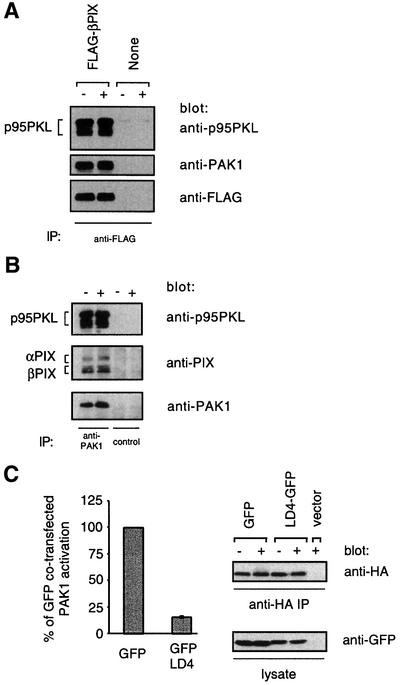
Fig. 6. p95PKL–PIX–PAK1 is required for efficient PAK1 activation by the TCR. (A) Either Jurkat cells stably expressing wild-type FLAG-βPIX or untransfected Jurkat cells were stimulated for 2 min with C305 (+) or a buffer control (–) and then lysed. Anti-FLAG immunoprecipitates were analyzed by 7.5% SDS–PAGE and blotting with an anti-p95PKL antibody (top panel) or an anti-PAK1 antibody (middle panel). The blot was then stripped and reprobed with an anti-FLAG antibody (bottom panel). These data are representative of two independent experiments. (B) Jurkat cells were stimulated for 2 min with C305 (+) or a buffer control (–) and then lysed. Endogenous PAK1 was immunoprecipitated from Jurkat cells with a C-terminally directed anti-PAK1 antibody (lanes 1 and 2) or the same antibody pre-incubated with a PAK1-blocking peptide (lanes 3 and 4). The immunoprecipitates were analyzed by 7.5% SDS–PAGE and western blotting with an anti-p95PKL antibody (top panel) and anti-PAK1 antibody (bottom panel). The top panel was stripped and reprobed with an anti-PIX antibody (middle panel), which explains the relatively weaker PIX signal. (C) Jurkat T cells were co-transfected with 15 µg of the GFP parental vector and 5 µg of pEF-HA-PAK1 or with 15 µg of LD4–GFP and 20 µg of pEF-HA-PAK1 to equalize expression of PAK1. Six hours after transfection, the cells were stimulated for 2 min with C305 or a buffer control at 37°C and PAK1 kinase activity was measured (left panel, n = 3). Equivalent immunoprecipitation of HA-PAK1 was confirmed by an anti-HA western blotting (right top panel) and expression of GFP and LD4–GFP was confirmed by an anti-GFP western blot from whole cell lysate (right bottom panel).
