FIG. 7.
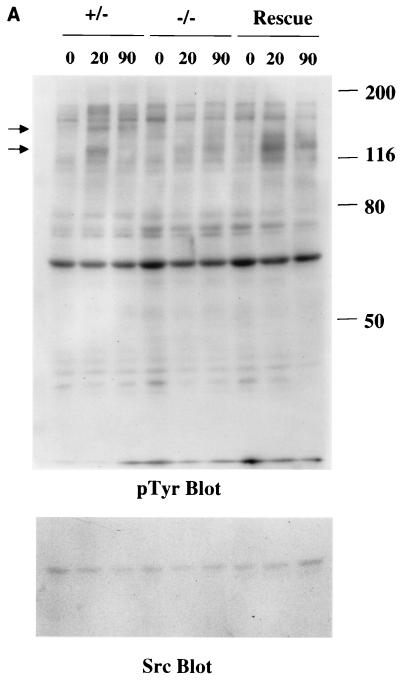
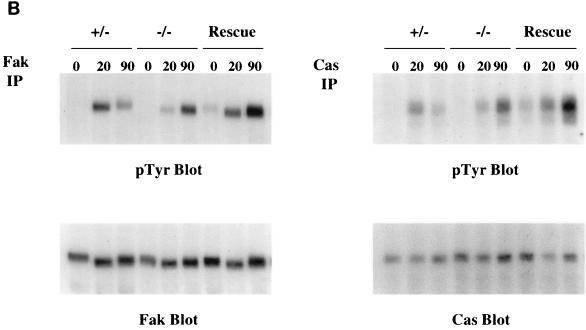
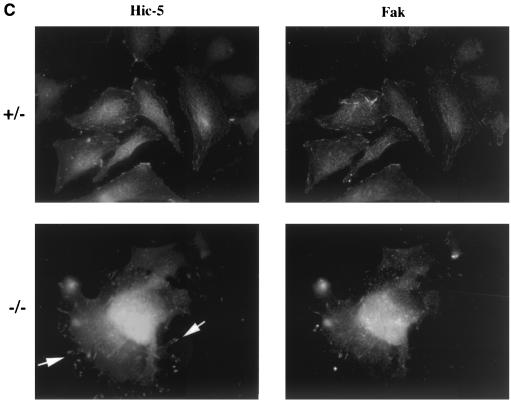
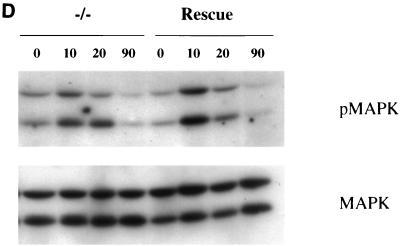
Fibronectin-induced phosphorylation of FAK, Cas, and MAPK and FAK localization are defective in paxillin-deficient cells. Cells were plated on fibronectin-coated plates for the indicated time points (in minutes). Lysates were prepared and analyzed for total cell phosphotyrosine (A) or tyrosine phosphorylation of specific substrates (B). The total cell lysate blot was probed with an antibody to Src to ensure equal loading. The pTyr blots of FAK and Cas immunoprecipitations were reprobed with an antibody to FAK or Cas to check for levels. (C) Cells were also costained with antibodies to FAK and Hic-5 to determine if loss of paxillin affects FAK localization. While Hic-5 and FAK colocalized to focal adhesions in control cells, a few Hic-5-positive focal adhesions which were negative for FAK in paxillin mutant cells were observed (arrows). (D) Total cell lysates were prepared from knockout or rescued cells plated on fibronectin for the indicated time points and analyzed with phospho-specific antibodies to MAPK. Blots were reprobed for MAPK to check for equal loading. In this experiment, comparison of the knockout cells to the rescued cells indicates that there is a twofold difference in MAPK activation at the 10- and 20-min time points.