Abstract
Among the tumor necrosis factor (TNF) family of cytokines, FasL and TNF-related apoptosis-inducing ligand (TRAIL) are known to induce cell death via caspase activation. Recently, other biological functions of these death ligands have been postulated in vitro and in vivo. It was previously shown that Fas ligation induces chemokine expression in human glioma cells. In this study, we investigated whether the TRAIL-DR5 system transduces signals similar to those induced by other TNF family ligands and receptors. To address this issue, two human glioma cell lines, CRT-MG and U87-MG, were used, and an agonistic antibody against DR5 (TRA-8) and human recombinant TRAIL were used to ligate DR5. We demonstrate that DR5 ligation by either TRAIL or TRA-8 induces two functional outcomes, apoptosis and expression of the chemokine interleukin-8 (IL-8); the nonspecific caspase inhibitor Boc-D-Fmk blocks both TRAIL-mediated cell death and IL-8 production; the caspase 3-specific inhibitor z-DEVD-Fmk suppresses TRAIL-mediated apoptosis but not IL-8 induction; caspase 1- and 8-specific inhibitors block both TRAIL-mediated cell death and IL-8 production; and DR5 ligation by TRAIL mediates AP-1 and NF-κB activation, which can be inhibited by caspase 1- and 8-specific inhibitors. These findings collectively indicate that DR5 ligation on human glioma cells leads to apoptosis and that the activation of AP-1 and NF-κB leads to the induction of IL-8 expression; these responses are dependent on caspase activation. Therefore, the TRAIL-DR5 system has a role not only as an inducer of apoptotic cell death but also as a tranducer for proinflammatory and angiogenic signals in human brain tumors.
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the TNF/NGF family of cytokines. Members of the TNF family of cytokines contain highly conserved carboxy-terminal domains and induce receptor trimerization to transduce signaling pathways (for a review, see reference 51). Members of the TNF/NGF family have been expanding and include TNF-α/β, lymphotoxin-β, FasL, TRAIL, TNF-related activation-induced cytokine, LIGHT, TWEAK, CD154, a proliferation-inducing ligand, TNF- and ApoL-related leukocyte-expressed ligand 1, CD137 ligand, CD134 ligand, and glucocorticoid-induced TNF receptor ligand (for a review, see reference 51). Among these members, FasL and TRAIL can induce apoptotic cell death through caspase-dependent mechanisms shared with the p55 TNF receptor. FasL and TRAIL are mainly expressed by cytotoxic T cells and NK cells and are involved in activation-induced apoptosis of T cells (29, 54). TRAIL binds four major different receptors, two of which, DR4 and DR5, can induce apoptosis; however, decoy receptors for TRAIL, DcR1 and DcR2, do not have the intracytoplasmic death domain to transduce apoptotic death signals, and they protect cells from TRAIL-mediated cell death by interfering with signaling through DR4 and DR5 (for a review, see reference 13). Interestingly, transformed tumor cells are generally more susceptible to TRAIL-mediated cell death due to the selective loss of decoy receptors (for a review, see reference 16).
Glioblastoma multiforme (GBM) is the most malignant and common brain tumor, comprising ∼23% of all primary brain tumors in adults. GBM tumors are refractory to all current therapeutic approaches, including surgery, radiotherapy, and chemotherapy (22). Human glioma cells express DR5 and undergo apoptosis upon TRAIL ligation in vitro (35, 53). Local injection of TRAIL exerted strong antitumor activity on intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity (37). In combination with conventional DNA-damaging chemotherapy, TRAIL showed synergistic cytotoxicity for human gliomas in vivo and in vitro (28). Therefore, systemic or intracranial TRAIL treatment may be a promising approach for human GBM tumors.
Interleukin-8 (IL-8), a member of the CXC family of chemokines, is a potent chemoattractant and activator of neutrophils and a strong angiogenic factor (3, 10, 21, 45, 46). Astrocytic expression of IL-8 is induced by various stimuli, such as lipopolysaccharide, IL-1β, and TNF-α (32). Furthermore, IL-8 is upregulated in astroglioma cell lines in response to ischemia or hypoxic conditions (9, 10). IL-8 is a significant angiogenic factor in various human cancers, including non-small-cell lung cancer, melanoma, GBM, and prostate cancer (9, 19, 42, 43). Passive immunization with neutralizing antibodies against IL-8 reduces tumorigenesis and angiogenic activity in human non-small-cell lung cancer in SCID mice (2). These results collectively suggest that tumor production of IL-8 may be crucial for the neovascularization necessary for tumor growth. Recently, it was reported that IL-8 is induced in human glioma cells upon ligation of Fas, a member of the TNF/NGF receptor family (7). This finding prompted us to study other members of the TNF/NGF family, such as TRAIL, not only as inducers of apoptosis but also as mediators of angiogenic factors in human brain tumors.
Both TRAIL and its receptors are expressed in human brain tumors (12, 30, 36, 37); however, little is known about the biological functions of the TRAIL-DR4 or -DR5 system in these tumors. In this study, we investigated the expression and function of the TRAIL-DR system in two human glioma cell lines, CRT-MG and U87-MG. We demonstrate that DR5 ligation not only leads to apoptosis of human glioma cells but also induces expression of the CXC chemokine IL-8. This response is dependent on TRAIL-induced caspase activation. To our knowledge, this is the first report to address an alternative role of the TRAIL-DR5 system in human glioma cell lines. These findings support the contention that the TNF/NGF family of cytokines and their receptors may play a role in tumor growth by inducing inflammatory and angiogenic mediators in human brain tumors.
MATERIALS AND METHODS
Cells and culture conditions.
CRT-MG human glioma cells were maintained in RPMI 1640 medium with 10 mM HEPES (pH 7.2) and 1 mM Earle’s balanced salt solution supplemented with 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml as previously described (7). U87-MG cells were grown in a 50:50 mixture of Dulbecco modified Eagle medium and Ham’s F-12 medium (Life Technologies, Gaithersburg, Md.) supplemented as described above (7).
Reagents.
Human recombinant IL-1β and TNF-α were purchased from Genzyme (Cambridge, Mass.). Human recombinant TRAIL (hrTRAIL) was purified as previously described (41) and was further purified by gel filtration chromatography. Mouse monoclonal anti-human DR5 antibody TRA-8 was generated as previously described (18). Goat anti-mouse immunoglobulin G1 (IgG1) antibody conjugated to phycoerythrin (PE) was purchased from Southern Biotechnology Associates (Birmingham, Ala.). Nonspecific caspase inhibitors, Boc-D-Fmk and z-VAD-Fmk, were purchased from Calbiochem (La Jolla, Calif.), as were caspase 1-, 2-, 3-, 4-, 5-, 6-, 8-, and 9-specific inhibitors (z-YVAD-Fmk, z-VDVAD-Fmk, z-DEVD-Fmk, Ac-LEVD-CHO, z-WEHD-Fmk, z-VEID-Fmk, z-IETD-Fmk, and z-LEHD-Fmk, respectively). The caspase 3 substrate, Ac-DEVD-AMC, was purchased from Alexis Biochemicals (San Diego, Calif.).
Total RNA isolation and RPA.
Cells were washed with ice-cold phosphate-buffered saline (PBS), and then RNA was extracted by using a method based on guanidinium isothiocyanate-phenol extraction followed by ethanol precipitation. A linearized human chemokine multiprobe set (hCK-5 45035P; PharMingen, San Diego, Calif.) was in vitro transcribed with T7 RNA polymerase, resulting in antisense RNA probes. An RNase protection assay (RPA) was carried out as described previously (7). Ten micrograms of total RNA was hybridized with the hCK-5 riboprobes. Values for chemokine mRNA levels were normalized to those for glyceraldehyde-3-phosphate dehydrogenase mRNA levels for each experimental condition.
Flow cytometric analysis.
Glioma cell lines (2 × 105 cells/well) were plated in six-well (35-mm2) plates (Costar, Cambridge, Mass.) and grown to 90% confluency. For analysis of DR5 protein expression, cells were trypsinized, suspended in PBS containing 5% fetal bovine serum and 0.02% azide, incubated with antibody TRA-8 (1 μg/ml), stained with PE-conjugated goat anti-mouse IgG antibody, washed twice, fixed in 1% paraformaldehyde, and then analyzed with FACStar (Becton Dickinson, Mountain View, Calif.). Negative controls were incubated with an isotype-matched (IgG1) control antibody and stained with goat anti-mouse IgG antibody conjugated to PE. Ten thousand cells were analyzed for each sample.
Western blotting.
Cell lysates were prepared as previously described (7), and 100 μg of protein was electrophoresed in a 10% sodium dodecyl sulfate gel. Proteins were then transferred to nitrocellulose and probed with mouse monoclonal anti-human TRAIL, caspase 1, and poly(ADP-ribose) polymerase (PARP) antibodies and rabbit polyclonal anti-human caspase 3 and 8 antibodies (PharMingen). Enhanced chemiluminescence was used for the detection of bound antibody.
Detection of cell death.
Cell death was determined by staining with Annexin V (PharMingen), a 35.8-kDa protein that has a strong affinity for phosphatidylserine. After treatment with antibody TRA-8 or hrTRAIL, cells were washed twice with PBS, trypsinized, suspended in 200 μl of binding buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 2.5 mM CaCl2, 1 mM MgCl2, 4% bovine serum albumin), and stained with 0.5 ng of Annexin V-fluorescein isothiocyanate (FITC) and 2.5 ng of propidium iodide (PI) as previously described (7). Ten thousand cells were analyzed with FACStar within 30 min after staining. Cell death, including apoptosis and necrosis, was defined as cell fractions stained with Annexin V and/or PI.
Caspase 3 activity measurement.
Cells were treated with antibody TRA-8 or hrTRAIL for various times and then lysed. Soluble lysates (20 μg of total protein) were incubated with 5 μg of the caspase 3 substrate, Ac-DEVD-AMC, in reaction buffer (100 mM NaCl, 50 mM HEPES, 10 mM dithiothreitol, 1 mM EDTA, 10% glycerol) for 2 h at 37°C. Samples were read on a fluorescence plate reader (Labtech, East Sussex, United Kingdom) at wavelengths of 360 nm for excitation and 460 nm for emission.
Plasmids and transfection.
The human IL-8 promoter-luciferase construct used for transient transfection was previously described (26), and the pCMV-β-galactosidase construct was purchased from Clontech (Palo Alto, Calif.). The human IL-8 promoter consists of 546 bp including AP-1-, NF-κB-, and NF-IL-6- responsive elements (26). Transient transfection of cell line CRT-MG was performed by electroporation with a Bio-Rad (Hercules, Calif.) gene pulser as previously described (7). After 36 h of recovery, cells were incubated in the presence of hrTRAIL for an additional 24 h. Cells were then harvested, and luciferase and β-galactosidase activities were measured. The luciferase activity of each sample was normalized to the β-galactosidase activity to calculate relative luciferase activity.
Stable reporter cell lines transfected with the human IL-8 promoter-luciferase reporter construct were generated. CRT-MG cells were transfected by electroporation as described above (7) and were maintained in medium containing 1.0 mg of G-418 (Life Technologies, Carlsbad, Calif.)/ml. Cells were grown in the absence of G-418 for 1 week before experiments. These stable IL-8 reporter cell lines showed a fold induction of luciferase activity in response to IL-1β, TNF-α, and hrTRAIL similar to that of cells transiently transfected with the human IL-8 promoter-luciferase construct (data not shown).
The IL-8 promoter-luciferase construct and a destabilized enhanced green fluorescent protein (EGFP)-expressing plasmid, pd2EGFP (Clontech), were digested by using XhoI and BamHI. The pd2EGFP backbone and the 546-bp insert containing the IL-8 promoter were ligated to generate IL-8p-d2EGFP. Construct IL-8p-d2EGFP was transfected as described above. Stable transfectants were grown in medium containing 0.5 mg of G-418/ml and cloned by using the automatic cell deposition unit (ACDU) of a FACSVantage instrument (Becton Dickinson). The expression of EGFP was confirmed by fluorescence-activated cell sorting (FACS) analysis. The CRT-MG transfectant used for the experiments described here was selected from more than 20 clones on the basis of a strong induction of IL-8 promoter activity in response to IL-1β and TNF-α, as assessed by FACS analysis (data not shown).
ELISA.
Cells were incubated in the absence or presence of Boc-D-Fmk for 3 h, followed by treatment with hrTRAIL for 36 h in complete medium. Concentrations of IL-8 in the supernatants were assayed by using a dual-antibody solid-phase enzyme-linked immunosorbent assay (ELISA) for IL-8 (Biosource International, Camarillo, Calif.). Cells were washed with PBS and lysed, and then the amount of total protein was measured by using a Bio-Rad protein assay kit. IL-8 expression was normalized to the amount of total protein.
EMSAs.
Nuclear extracts were prepared from cells treated with hrTRAIL in the absence or presence of various caspase inhibitors as described previously (31). AP-1 and NF-κB consensus oligonucleotides (Santa Cruz, Santa Cruz, Calif.) used in electrophoretic mobility shift assays (EMSAs) were 5′-CGCTTGATGACTCAGCCGGAA-3′ and 5′-AGTTGAGGGGACTTTCCCAGG-3′, respectively. Complementary oligonucleotides were annealed and end labeled with [α-32P]ATP by using T4 polynucleotide kinase. EMSAs were performed with a total reaction volume of 20 μl at 4°C. Five micrograms of nuclear extracts was equilibrated for 15 min in binding buffer (10 mM Tris-HCl [pH 8.0], 75 mM KCl, 2.5 mM MgCl2, 0.1 mM EDTA, 10% glycerol, 0.25 mM dithiothreitol) with 1 μg of poly(dI/dC). A 32P-labeled oligonucleotide probe (20,000 cpm) was then added, and the reaction mixture was incubated on ice for an additional 20 min. Bound and free DNAs were then resolved by electrophoresis on a 5% native polyacrylamide gel in TBE buffer (89 mM Tris-HCl, 89 mM boric acid, 2 mM EDTA).
Statistical analysis.
Data represent the mean and standard deviation (SD). Levels of significance for comparisons between samples were determined by using the Student t test distribution.
RESULTS
Expression of DR5 and TRAIL by human gliomas.
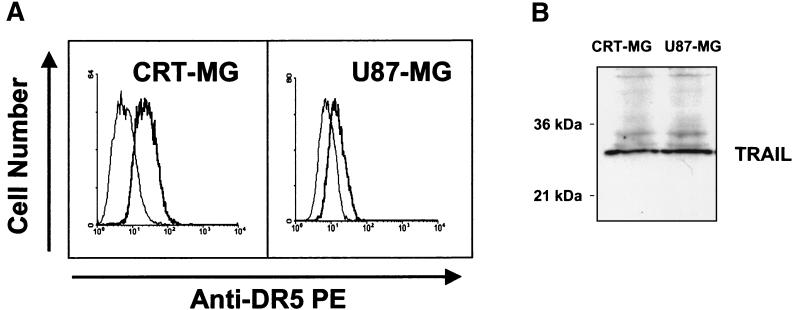
It was previously shown that the human glioma cell lines U87-MG and CRT-MG constitutively express Fas, although sensitivity to Fas-mediated cell death is variable (7). U87-MG glioma cells are susceptible to Fas-mediated cell death, while CRT-MG cells are resistant to Fas-mediated apoptosis. To start our examination of TRAIL and its receptors in glioma cells, we examined the mRNA expression of TRAIL and its receptors (DR4, DR5, and DcR1) by using an RPA. CRT-MG and U87-MG cells expressed mRNA for DR5 and DcR1 as well as TRAIL, while DR4 mRNA was not detected (data not shown). Protein expression of DR5 and TRAIL was investigated by FACS analysis and Western blotting, respectively. As shown in Fig. 1, CRT-MG and U87-MG cells constitutively expressed both DR5 and TRAIL proteins.
FIG. 1.
Expression of DR5 and TRAIL by human astroglioma cells. (A) FACS analysis of DR5 expression on CRT-MG and U87-MG cells was performed as described in Materials and Methods. Thin lines indicate negative controls stained with secondary antibody conjugated to PE after incubation with an isotype-matched (IgG1) control antibody. (B) Cell lysates were prepared from CRT-MG and U87-MG cells, and 100 μg of protein was electrophoresed in 10% sodium dodecyl sulfate gels. Western blot analysis for TRAIL protein expression was done as described in Materials and Methods. The data are representative of three independent experiments.
TRAIL and TRA-8 induce apoptotic cell death and caspase 3 activation.
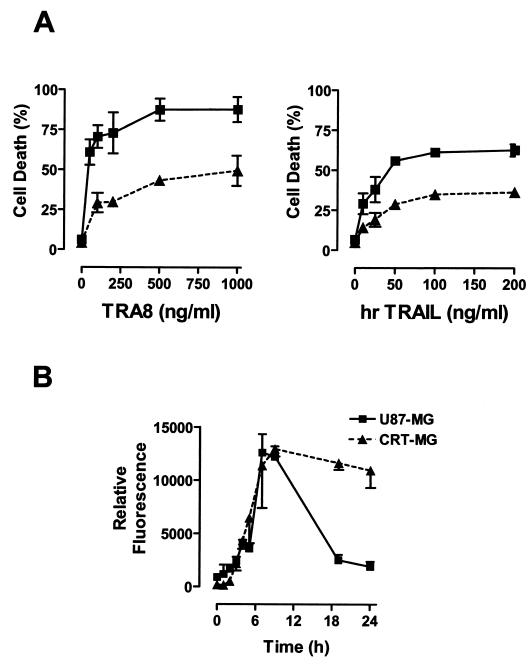
To investigate susceptibility to TRAIL-mediated cell death, CRT-MG and U87-MG cells were incubated with either mouse anti-human DR5 monoclonal antibody TRA-8 or hrTRAIL, and then apoptotic cell death was measured by FACS analysis after staining with Annexin V-FITC and PI. TRA-8 exclusively recognizes DR5 (18), while hrTRAIL has the capacity to bind all the DR receptors (16). TRA-8 and hrTRAIL induced the death of both human glioma cell lines in a dose-dependent manner (Fig. 2A), with TRA-8 having a stronger potency. U87-MG cells were more sensitive to both TRA-8- and hrTRAIL-mediated cell death than CRT-MG cells.
FIG. 2.
Susceptibility to DR5-induced cell death and activation of caspase 3. (A) U87-MG (▪) and CRT-MG (▴) cells were incubated with various concentrations of anti-DR5 antibody TRA-8 (0 to 1,000 ng/ml) or hrTRAIL (0 to 200 ng/ml) for 24 h, and then cell death was measured by staining with Annexin V-FITC and PI. Data are the mean and SD for duplicate samples from one experiment and are representative of three independent experiments. (B) Cells were incubated with TRA-8 (100 ng/ml) in complete medium for various times (0 to 24 h), and caspase 3 activity was measured as indicated in Materials and Methods. The relative fluorescence of each sample was normalized to the amount of total protein. Data are the mean and SD for quadruplicate samples from one experiment and are representative of two independent experiments.
To determine whether caspase activation is involved in DR5-mediated cell death, we examined the in vitro enzyme activity of the downstream caspase, caspase 3, upon DR5 engagement. Cells were treated with TRA-8 (100 ng/ml) for various times, and then the activity of caspase 3 in cell lysates was measured by proteolytic digestion of a caspase 3-specific fluorogenic substrate, Ac-DEVD-AMC. As shown in Fig. 2B, in vitro caspase 3 activity reached maximal levels 6 to 10 h after DR5 ligation and was maintained until 24 h in CRT-MG cells but declined in U87-MG cells. These results indicate that CRT-MG and U87-MG cells are sensitive to DR5-mediated cell death, which is preceded by caspase 3 activation.
DR5 ligation induces IL-8 expression.
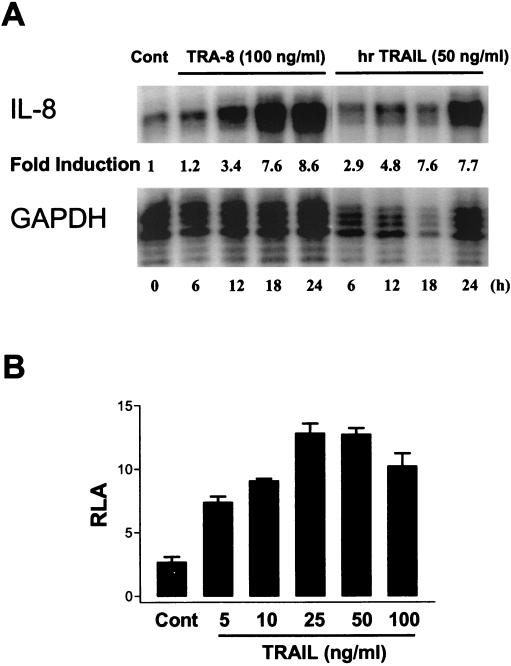
We next examined if engagement of DR5 leads to functional outcomes other than apoptosis. CRT-MG and U87-MG cells were incubated with antibody TRA-8 (100 ng/ml) or hrTRAIL (50 ng/ml) for various times (0 to 24 h), and RNA was extracted and then analyzed by an RPA for chemokine mRNA expression. IL-8 was constitutively expressed in CRT-MG cells, and treatment with TRA-8 or hrTRAIL led to the selective enhancement of IL-8 mRNA expression in a time-dependent manner (Fig. 3A). Monocyte chemoattractant protein 1 (MCP-1) was also constitutively expressed, but TRA-8 or hrTRAIL treatment did not enhance expression (data not shown). Lymphotactin, gamma interferon-inducible protein 10, macrophage inflammatory protein 1α/β, and I-309 were not expressed basally or induced upon DR5 ligation (data not shown). Optimal IL-8 mRNA expression was observed at 18 to 24 h (Fig. 3A), which is also the time at which CRT-MG cells undergo apoptosis (∼30% cell death) (Fig. 2A). Treatment with TRA-8 or hrTRAIL also selectively enhanced IL-8 mRNA expression in U87-MG cells (data not shown).
FIG. 3.
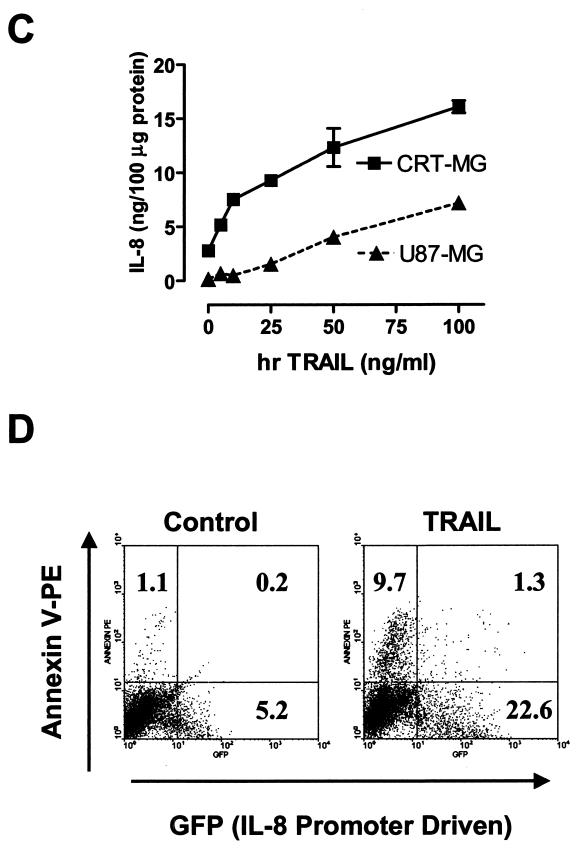
Induction of IL-8 expression upon DR5 ligation. (A) CRT-MG cells were treated with TRA-8 (100 ng/ml) or hrTRAIL (50 ng/ml) for various times (0 to 24 h), and IL-8 mRNA expression was measured by an RPA. Fold induction of IL-8 mRNA expression was normalized to the level of glyceraldehyde-3-phosphate dehydrogenase mRNA expression. The data are representative of three independent experiments. Cont, control. (B) CRT-MG cells were transiently transfected with an IL-8 promoter-luciferase construct and the pCMV-β-galactosidase construct as indicated in Materials and Methods. The cells were incubated with hrTRAIL (0 to 100 ng/ml) for 24 h, harvested, and then examined for luciferase and β-galactosidase activities. The luciferase activity of each sample was normalized to the β-galactosidase activity, and fold induction values were calculated as the ratio of the normalized luciferase activity of each sample to that of the control. RLA, relative luciferase activity. Data are the mean and SD for duplicate samples from one experiment and are representative of three experiments. (C) CRT-MG and U87-MG cells were treated with hrTRAIL (0 to 100 ng/ml) for 36 h, and supernatants were examined for IL-8 protein expression by an ELISA. IL-8 expression was normalized to the amount of total protein. Data are the mean and SD for duplicate samples from one experiment and are representative of three independent experiments. (D) CRT-MG cells stably transfected with construct IL-8p-d2EGFP were treated with hrTRAIL (50 ng/ml) for 36 h, stained with Annexin-V-PE, and then analyzed by FACS. The percentage of cells in each quadrant is indicated. GFP, green fluorescent protein. The data are representative of three independent experiments.
To determine if DR5 ligation affects IL-8 expression at the transcriptional level, IL-8 promoter activity was examined. CRT-MG cells were transiently transfected with an IL-8 promoter-luciferase construct (26) and the pCMV-β-galactosidase construct to monitor transfection efficiency and then were incubated with increasing concentrations of hrTRAIL (0 to 100 ng/ml) for 24 h. TRAIL ligation of DR5 induced IL-8 luciferase reporter activity in a dose-dependent manner (∼5-fold induction with 25 to 50 ng of hrTRAIL/ml) (Fig. 3B). Treatment with TRA-8 also induced IL-8 promoter activity in CRT-MG cells in a similar pattern (data not shown).
We next examined IL-8 protein expression upon DR5 ligation. CRT-MG and U87-MG cells were incubated with increasing concentrations of hrTRAIL (0 to 100 ng/ml) for 36 h, and then supernatants were harvested and analyzed by an ELISA. It should be noted that IL-8 protein expression was normalized to the amount of total protein, to take into consideration the cell death also occurring in these cells. As shown in Fig. 3C, IL-8 protein production occurred in a dose-dependent manner upon incubation with hrTRAIL in both CRT-MG and U87-MG cells, with CRT-MG cells producing more IL-8. Antibody TRA-8 also induced IL-8 protein expression in a similar manner (data not shown). These results collectively indicate that ligation of DR5 on glioma cells leads to the induction of IL-8 mRNA and protein expression and that this induction is mediated at the transcriptional level.
DR5 ligation induces both apoptosis and IL-8 expression in human glioma cells; however, the question was raised as to whether these two functional outcomes of DR5 ligation happen in identical or different sets of cells. To address this question, we generated CRT-MG and U87-MG cells stably transfected with construct IL-8p-d2EGFP as described in Materials and Methods. Transfected cells express destablized EGFP under the control of the IL-8 promoter (half life, ∼3 h). These stable transfectants were treated with hrTRAIL (50 ng/ml) for 36 h, stained with Annexin V-PE, and then analyzed by using FACS to determine the degree of apoptosis (Annexin V positivity) and IL-8 promoter activity (EGFP expression) simultaneously (Fig. 3D). A low level of basal expression of EGFP was observed in unstimulated cells (5.2%; mean fluorescence intensity [MFI], 30.1); this expression correlated with the basal levels of mRNA and protein expression of IL-8 (Fig. 3A and C). As expected, treatment with hrTRAIL increased the percentage (23.9%) and MFI (112.0) of EGFP-expressing cells (Fig. 3D). At the same time, hrTRAIL increased the amount of Annexin V-PE-positive cells; however, these apoptotic cells expressed a lower level of EGFP (MFI, 46.4) than did live cells (Annexin V-PE-negative cells). These results indicate that both functional outcomes of DR5 ligation, apoptosis and IL-8 expression, occur in a mutually exclusive manner in human glioma cells.
Nonspecific caspase inhibition suppresses both apoptosis and IL-8 induction upon DR5 ligation.
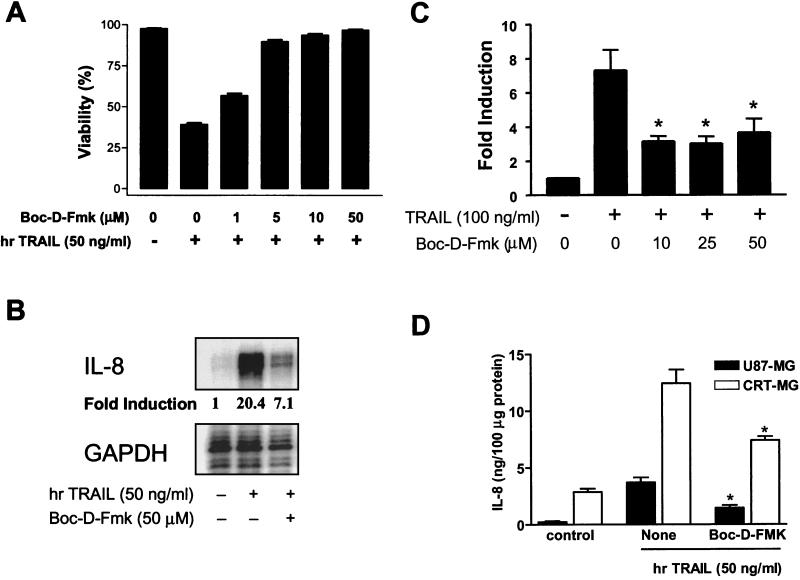
We have shown that caspase 3 activation precedes DR5-mediated cell death, so we wished to determine if caspase activation is responsible for cell death. Cells were pretreated with the cell-permeable nonspecific caspase inhibitor Boc-D-Fmk (0 to 50 μM) for 3 h, followed by treatment with hrTRAIL (50 ng/ml) for an additional 24 h, and total cell death was measured as indicated in Materials and Methods. As shown in Fig. 4A, treatment with Boc-D-Fmk suppressed TRAIL-induced cell death in a dose-dependent manner. Another nonspecific caspase inhibitor, z-VAD-Fmk (50 μM), also inhibited DR5-induced cell death (data not shown). These results collectively indicate that caspases are involved in DR5-induced cell death.
FIG. 4.
Effect of the nonspecific caspase inhibitor Boc-D-Fmk on apoptosis and IL-8 induction upon DR5 ligation. (A) CRT-MG cells were incubated in the absence or presence of the cell-permeable nonspecific caspase inhibitor Boc-D-Fmk (0 to 50 μM) for 3 h prior to a 24-h treatment with hrTRAIL (50 ng/ml), and cell death was evaluated by staining with Annexin-V and PI as indicated in Materials and Methods. Data are the mean and SD for duplicate samples from one experiment and are representative of three independent experiments. (B) CRT-MG cells were pretreated in the absence (second lane) or presence (third lane) of Boc-D-Fmk (50 μM) for 3 h, washed twice, and then treated with hrTRAIL (50 ng/ml) for an additional 24 h. IL-8 mRNA expression was measured by an RPA; fold induction of IL-8 is indicated. Data are representative of three experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C) CRT-MG cells were stably transfected with construct IL-8p-d2EGFP as indicated in Materials and Methods. The stable reporter cells were incubated in the absence or presence of Boc-D-Fmk (0 to 50 μM) for 3 h and treated with hrTRAIL (100 ng/ml) for an additional 24 h, and then EGFP expression was examined by FACS analysis. Data are the mean and SD for duplicate samples from three independent experiments. The asterisk indicates a P value of ≤0.05. (D) Cells were incubated in the absence or presence of Boc-D-Fmk (50 μM) for 3 h and washed twice prior to a 36-h treatment with hrTRAIL (50 ng/ml), and IL-8 protein expression was measured by an ELISA. Data are the mean and SD for quadruplicate samples from one experiment. Values significantly different from the values for the hrTRAIL-treated sample without Boc-D-Fmk pretreatment are indicated by an asterisk (P ≤ 0.05). The data are representative of two independent experiments.
Because caspase inhibition blocked DR5-induced apoptosis, we speculated that this effect might increase DR5-induced IL-8 expression by rescuing dying cells and increasing the live-cell portion that can produce IL-8. To address this notion, cells were incubated in the absence or presence of Boc-D-Fmk (50 μM) for 3 h, washed twice, and treated with hrTRAIL (50 ng/ml) for an additional 24 h; then, an RPA was performed for the analysis of IL-8 mRNA expression. Preincubation with Boc-D-Fmk partially inhibited (∼66%) DR5-induced IL-8 mRNA expression (Fig. 4B). Comparable results were obtained when we examined the influence of Boc-D-Fmk on DR5-induced IL-8 promoter activity and protein production (Fig. 4C and D). Another nonspecific caspase inhibitor, z-VAD-Fmk, also inhibited DR5-induced IL-8 mRNA and protein expression (data not shown). These results indicate that caspase activation is required for both DR5-mediated apoptosis and IL-8 expression.
Caspase inhibition differentially blocks apoptosis and IL-8 induction upon TRAIL ligation.
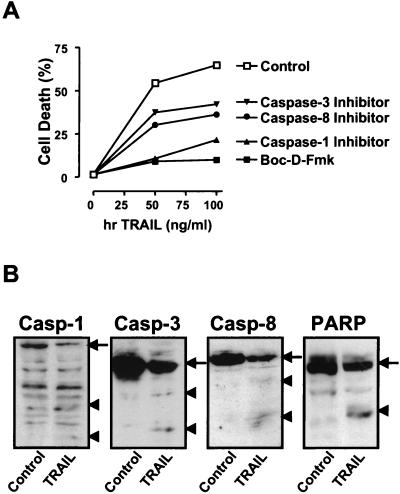
Nonspecific caspase inhibitors suppressed both TRAIL-mediated apoptosis and IL-8 production. Thus, we next determined which caspases are involved in the different signaling pathways. Cells were pretreated with various cell-permeable caspase inhibitors (10 μM) for 3 h, followed by treatment with hrTRAIL (0 to 100 ng/ml) for an additional 24 h, and total cell death was measured as indicated in Materials and Methods (Fig. 5A). Treatment with Boc-D-Fmk completely blocked TRAIL-mediated apoptosis, while caspase 3- and 8-specific inhibitors suppressed ∼50% of cell death. Surprisingly, the caspase 1-specific inhibitor also blocked ∼75% of TRAIL-mediated cell death. Other inhibitors of caspases (caspases 2, 4, 5, and 9) had no inhibitory effect on TRAIL-mediated cell death (data not shown). These results suggest that caspases 1, 3, and 8 are responsible for DR5-mediated cell death. We further investigated whether these caspases were proteolytically cleaved to produce active proteases upon TRAIL ligation. To address this issue, cells were incubated with hrTRAIL (100 ng/ml) for 24 h, and lysates were examined by Western blot analysis as described in Materials and Methods (Fig. 5B). Treatment with hrTRAIL diminished the amounts of the full-length precursors of caspases 1, 3, and 8 and resulted in active caspase 1, 3, and 8 products. PARP, a 116-kDa nuclear chromatin-associated enzyme and a well-known target of active caspases (5), was cleaved into an 85-kDa fragment upon hrTRAIL treatment (Fig. 5B). These results collectively indicate that caspases 1, 3, and 8 are proteolytically processed and mediate DR5-mediated cell death in human astroglioma cells.
FIG. 5.
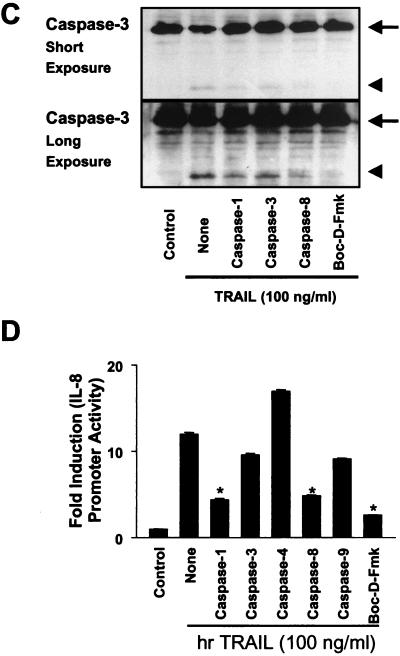
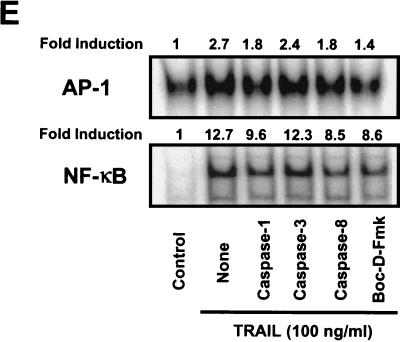
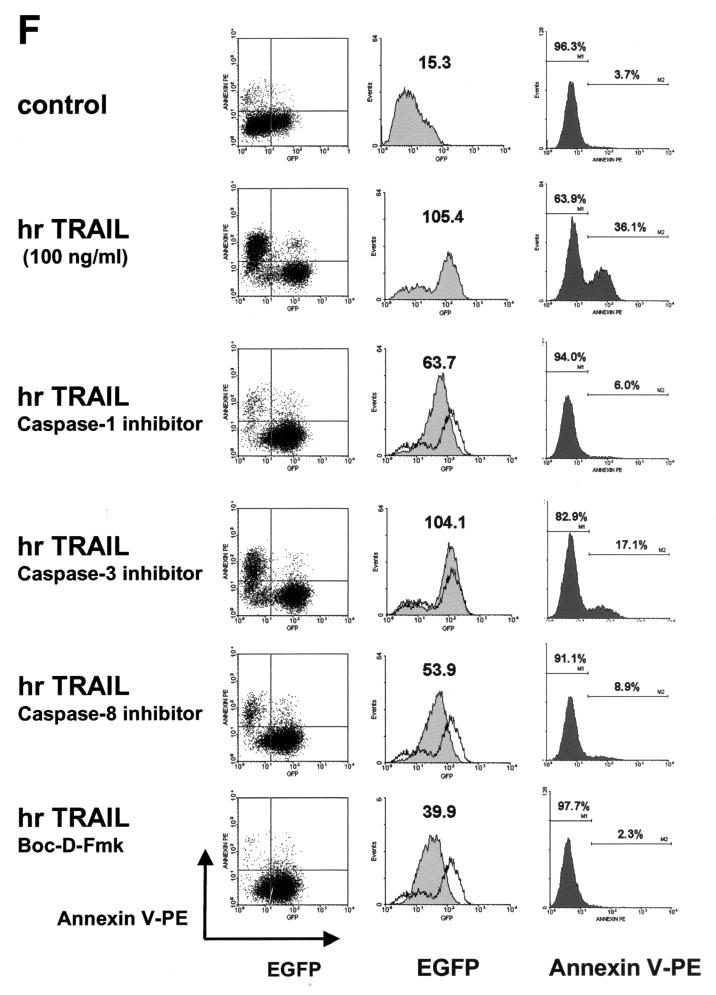
Differential effects of caspase inhibitors on TRAIL-mediated apoptosis and IL-8 induction. (A) CRT-MG cells were incubated in the absence or presence of various caspase inhibitors (10 μM) for 3 h and treated with hrTRAIL (0 to 100 ng/ml) for 24 h, and then cell death was measured by staining with Annexin V-FITC and PI. The data are representative of three independent experiments. (B) CRT-MG cells were incubated with medium or hrTRAIL (100 ng/ml) for 24 h, and 40 μg of lysates was examined by Western blot analysis with antibodies against caspases (Casp) 1, 3, and 8 and PARP. The arrows indicate the precursors of caspases 1 (45 kDa), 3 (32 kDa), and 8 (55 kDa) and PARP (116 kDa). The arrowheads indicate proteolytic products of caspases 1 (20 and 30 kDa), 3 (17 and 22 kDa), and 8 (23 and 40 kDa) and PARP (85 kDa). The data are representative of three independent experiments. (C) CRT-MG cells were incubated in the absence or presence of various caspase inhibitors (50 μM) for 3 h and treated with hrTRAIL (100 ng/ml) for 24 h, and then 40 μg of lysates was examined by caspase 3 Western blot analysis. The upper panel indicates short-term exposure of the blot, and the lower panel shows prolonged exposure of the same blot. The arrows indicate unprocessed procaspase 3 (32 kDa), and the arrowheads indicate processed active caspase 3 products (17 kDa). The data are representative of three independent experiments. (D) CRT-MG cells stably transfected with the IL-8 promoter-luciferase construct were incubated in the absence or presence of various caspase inhibitors (10 μM) for 3 h and treated with hrTRAIL (100 ng/ml) for 24 h, and then lysates were examined for luciferase activity. The luciferase activity of each sample was normalized to the amount of total protein, and fold induction values were calculated as the ratio of the luciferase activity of each sample normalized to that of the control. Data are the mean and SD for duplicate samples from one experiment. Values significantly different from values for the hrTRAIL-treated sample without inhibitor treatment are indicated by asterisks (P ≤ 0.05). The data are representative of three experiments. (E) CRT-MG cells were incubated in the absence or presence of various caspase inhibitors (10 μM) for 3 h and treated withhrTRAIL (100 ng/ml) for 1 h, and then 5 μg of nuclear extracts was examined for AP-1 and NF-κB binding by an EMSA as indicated in Materials and Methods. Fold induction values were calculated as the ratio of the value for each sample to that for the control. The data are representative of three independent experiments. (F) An CRT-MG cell clone stably transfected with construct IL-8p-d2EGFP was incubated in the absence or presence of various caspase inhibitors (25 μM) for 3 h, treated with hrTRAIL (100 ng/ml) for 24 h, and analyzed by FACS after staining with Annexin V-PE. The diagrams on the left indicate the results of FACS: EGFP expression (IL-8 promoter driven) versus Annexin V-PE (apoptosis). The histograms in the middle indicate the EGFP expression of living cells (after selection of Annexin V-PE-negative cells). The MFI is indicated. The histograms on the right show Annexin V-PE staining. The data are representative of three experiments.
Next, we examined whether the activation of caspase 3 upon DR5 ligation could also be blocked by caspase inhibitors. Proteolytic degradation of caspase 3 upon TRAIL ligation was performed by using Western blot analysis as described in Materials and Methods. Cells were incubated in the absence or presence of various caspase inhibitors (50 μM) for 3 h and treated with hrTRAIL (100 ng/ml) for 24 h, and Western blot analysis was performed with a caspase 3-specific antibody. As shown in Fig. 5C, treatment with hrTRAIL induced the degradation of the caspase 3 precursor (upper panel) into active caspase 3 products (lower panel). Pretreatment with Boc-D-Fmk completely blocked DR5-mediated processing of the caspase 3 precursor. Caspase 1-, 3-, and 8-specific inhibitors seemed to protect the caspase 3 precursor from DR5-mediated degradation (Fig. 5C, upper panel); however, active proteolytic products of caspase 3 were observed after prolonged exposure of the blot, suggesting that the inhibition of caspase 3 proteolysis by caspase 1-, 3-, and 8-specific inhibitors was incomplete (lower panel). These results collectively indicate that caspases 1, 3, and 8 are involved in DR5-mediated caspase 3 activation and apoptosis.
To determine which caspases are involved in DR5-mediated IL-8 induction, stable reporter cells transfected with the IL-8 promoter-luciferase construct were incubated in the absence or presence of various caspase inhibitors (10 μM) for 3 h and treated with hrTRAIL (100 ng/ml) for an additional 24 h, and then lysates were assayed for luciferase activity (Fig. 5D). Preincubation with Boc-D-Fmk inhibited ∼90% of TRAIL-induced IL-8 promoter activity, while caspase 1- and 8-specific inhibitors blocked ∼55% of TRAIL-mediated IL-8 promoter activity. However, caspase 3- and 9-specific inhibitors did not significantly affect TRAIL-induced IL-8 promoter activity, while the caspase 4-specific inhibitor enhanced promoter activity. These results suggest that caspases 1 and 8 are involved in both TRAIL-mediated apoptosis and IL-8 production, while caspase 3 is involved in TRAIL-mediated apoptosis but not TRAIL-induced IL-8 production.
Transcriptional regulation of the IL-8 gene has been well documented for various cell types, and NF-κB and AP-1 are the major transcription factors involved in IL-8 gene expression (26). In this study, we showed that TRAIL ligation induces IL-8 expression at the transcriptional level (Fig. 3B); thus, we hypothesized that TRAIL ligation may also activate AP-1 and NF-κB. To assess this notion, an EMSA was performed by using AP-1 and NF-κB consensus oligonucleotides as indicated in Materials and Methods. CRT-MG cells were incubated with hrTRAIL (100 ng/ml) for 1 h, and then nuclear extracts were assayed for AP-1 and NF-κB binding (Fig. 5E). TRAIL ligation induced NF-κB binding (12.7-fold induction) and modestly enhanced AP-1 binding (2.7-fold). The influence of various caspase inhibitors was next examined. Cells were pretreated with the caspase inhibitors (10 μM) for 3 h and then exposed to hrTRAIL for an additional 1 h. TRAIL-mediated AP-1 and NF-κB binding was partially inhibited by Boc-D-Fmk (∼77 and ∼40%, respectively). Caspase 1- and 8-specific inhibitors suppressed ∼55% of AP-1 and ∼40% of NF-κB binding, while the caspase 3-specific inhibitor had no effect on DR5-mediated AP-1 and NF-κB activation. These results indicate that DR5 ligation affects the binding activities of AP-1 and NF-κB, two transcription factors important for IL-8 gene expression, and that this response is dependent on caspase activation, especially that of caspases 1 and 8.
A CRT-MG cell clone stably transfected with construct IL-8p-d2EGFP was selected to study two functional outcomes simultaneously: apoptosis and IL-8 expression. The stably transfected cells were incubated with various caspase inhibitors (25 μM) for 3 h, followed by treatment with hrTRAIL (100 ng/ml) for an additional 24 h, and cell death and IL-8 promoter activity were assayed (Fig. 5F). Unstimulated cells expressed a low level of EGFP and did not undergo apoptosis spontaneously. After 24 h of TRAIL treatment, cells underwent apoptosis (36.1%) or expressed a higher level of EGFP (∼7-fold induction) than did the control sample. Pretreatment with Boc-D-Fmk decreased both apoptosis (∼100% inhibition) and EGFP expression (∼63% inhibition). Similarly, pretreatment with the caspase 1- or 8-specific inhibitor suppressed ∼92% of apoptosis and ∼50% of EGFP expression. However, the caspase 3-specific inhibitor blocked ∼53% of apoptosis but had no effect on EGFP expression. These results are consistent with previous findings and confirm that caspases 1, 3, and 8 are differentially involved in TRAIL-mediated apoptosis and IL-8 expression.
DISCUSSION
In this study, we show for the first time an alternative function of the TRAIL-DR5 system other than induction of apoptosis. Using the human GBM cell lines CRT-MG and U87-MG, we demonstrate that ligation of DR5 by hrTRAIL or the agonistic anti-DR5 antibody TRA-8 selectively induces the expression of CXC chemokine IL-8. IL-8 induction is seen only in the subpopulation of GBM cells resistant to DR5-induced apoptosis. DR5-induced apoptosis and IL-8 expression are mediated by caspase-dependent pathways.
Although the FasL-Fas system has been reported to induce proinflammatory mediators, such as IL-8 and MCP-1 (7), little is known about the immunological function of the TRAIL-DR system other than as an inducer of apoptosis. Even though the TRAIL-DR5 system evokes responses in human glioma cells similar to those evoked by the FasL-Fas system, there are several important differences between the two systems. First, DR5 ligation is able to induce the death of both CRT-MG and U87-MG cells, while Fas ligation induces the death of only U87-MG cells. Second, Fas ligation induces IL-8 and MCP-1 expression; however, DR5 ligation induces only IL-8 expression. Third, Fas ligation induces maximal levels of IL-8 mRNA within 3 h, while optimal expression of IL-8 mRNA upon DR5 ligation occurs at 18 to 24 h. Thus, there are clearly different intracellular events occuring upon activation of Fas and DR receptors in human glioma cells.
While IL-8 was initially discovered on the basis of chemotaxis and activation of neutrophils, IL-8 also has potent angiogenic properties (21). Members of the CXC chemokine family are polypeptide molecules ranging from 7 to 10 kDa and displaying four highly conserved cysteine amino acid residues, with the first two cysteines being separated by a nonconserved amino acid residue (the CXC cysteine motif). CXC chemokines that contain the three-amino-acid sequence Glu-Leu-Arg (the ELR motif), such as IL-8, are potent inducers of angiogenic activity relative to members that lack the ELR motif (47). In addition, CXC chemokines that lack the ELR motif, such as platelet factor 4, gamma interferon-inducible protein 10, and a monokine induced by gamma interferon, are potent inhibitors of both CXC chemokine- and basic fibroblast growth factor-induced angiogenesis (47). IL-8 is a significant angiogenic factor found in various human tumors, including GBM tumors (7, 9, 19, 42, 43). Tumor cells may be protected from Fas- or DR5-mediated apoptosis by the opposing angiogenic activity of IL-8 induced by Fas and DR5 ligation. However, previous reports suggested that the effects of IL-8 on tumorigenesis are not due to IL-8 functioning as an autocrine growth factor for tumor cells but are primarily due to angiogenic actions on surrounding endothelial cells (2). IL-8 binds to CXC chemokine receptors 1 and 2 (CXCR1 and CXCR2). CXCR2 is expressed by human microvascular endothelial cells (39) and is responsible for CXC chemokine-mediated angiogenesis (1). Human glioma cell lines CRT-MG and U87-MG do not constitutively express either CXCR1 or CXCR2 (data not shown); thus, it is unlikely that IL-8 directly affects these cells. It has been reported that IL-8 suppresses Fas-mediated apoptosis in human fetal astrocytes (38). Therefore, cells may be rescued from Fas- or DR5-mediated apoptosis by the direct protective properties of IL-8. However, the paucity of CXCR1 and CXCR2 expression on human glioma cells renders this possibility unlikely. Incubation with human recombinant IL-8 or neutralizing anti-IL-8 antibody had no effect on the Fas- or DR5-mediated death of human glioma cells (data not shown). The antiapoptotic effect of IL-8 may be restricted to early-passage primary astrocytes, perhaps due to different environmental cues (38).
We have demonstrated that DR5-mediated IL-8 induction and apoptosis are dependent on caspase activation by showing that caspase inhibitors block DR5-induced IL-8 expression and apoptosis. Protection from DR5-mediated apoptosis by caspase inhibitors was expected and is consistent with previous reports (for a review, see reference 51). However, suppression of DR5-induced IL-8 expression by caspase inhibitors was an unexpected finding, since we anticipated that inhibition of apoptosis might increase IL-8 expression by driving DR5-mediated signals to IL-8 induction. The partial (∼50 to 60%) inhibition of IL-8 expression by Boc-D-Fmk does suggest that a non-caspase-dependent pathway is activated by DR5 ligation.
The signal transduction pathways of Fas- and DR5-induced apoptosis share the common steps of recruitment of FADD and caspase 8 upon receptor trimerization (20, 44). In addition to caspase 8, caspases 3 and 10 have been reported to be involved in DR5-mediated cell death for various cell types (20, 44). Caspases 8 and 10 are known as apoptotic initiators, while caspase 3 is the major apoptotic executioner (for a review, see references 5, 15, and 50). Our finding that DR5-mediated apoptosis is completely suppressed by nonspecific caspase inhibitors but is inhibited only ∼50% by a caspase 3-specific inhibitor suggests that other executioner caspases, such as caspases 6 and/or 7, may be involved in DR5-mediated apoptosis.
Our data also indicate that caspase 1 is involved in DR5-mediated cell death in human glioma cells, along with caspases 3 and 8. Caspase 1 was first identified as the IL-1β-converting enzyme, also capable of processing IL-18, and is now classified as a cytokine processor caspase, along with caspases 4, 5, and 11 (11). Even though the major role of caspase 1 has been to mediate cytokine processing, a role for caspase 1 as an inducer of apoptosis has also been postulated. Caspase 1 knockout animals develop normally and have normal phenotypes (25); however, the progression of neurodegeneration in amyotrophic lateral sclerosis and Huntington’s disease models is much slower in these animals (23, 33). It has also been shown that the prodomain of caspase 1 is translocated into the nucleus and enhances Fas-mediated death signals through caspase 8 activation (49). However, DR5-induced apoptosis appears to be mediated not by the direct action of the prodomain of caspase 1 but rather by the proteolytic activity of caspase 1, since the active-site inhibitor, z-YVAD-Fmk, blocked DR5-mediated apoptosis. We have demonstrated that caspase 1, along with caspases 3 and 8, is involved in DR5-mediated cell death for human glioma cells; however, the molecular mechanism must be investigated further.
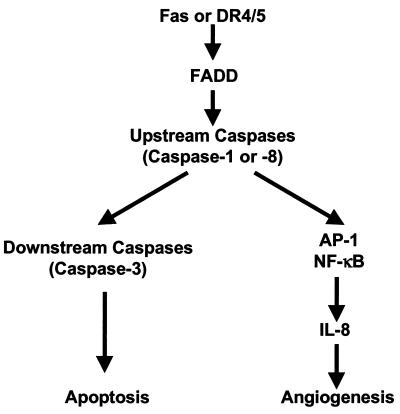
Our results indicate that the signal transduction pathways responsible for DR5-mediated apoptosis and IL-8 induction have common steps, at least at the level of upstream caspases (caspases 1 and 8), from which signal transduction pathways for apoptosis or IL-8 induction diverge (Fig. 6). Certain caspases (caspases 1 and 4) have been shown to process cytokines, such as IL-1β and IL-18 (11). A role of IL-1β and/or IL-18 as intermediate inducers for DR5-mediated IL-8 expression is a possibility. To address this notion, cells were preincubated with an IL-1 receptor antagonist prior to DR5 ligation; such treatment did not affect DR5-mediated IL-8 expression, suggesting that IL-1 is not involved in this response (data not shown). Regarding IL-18, neither IL-18 nor the IL-18 receptor is detected in human astroglioma cell lines (CRT-MG and U87-MG), as determined by ELISA, Western blot, and FACS analyses (data not shown). These results indicate that IL-18 does not play a role in DR5-mediated IL-8 expression.
FIG. 6.
Proposed model for signal transduction pathways upon death receptor ligation.
Our results demonstrate that DR5-induced IL-8 expression occurs at the level of promoter activation. Transcriptional regulation of IL-8 expression has been well documented for various cell types. NF-κB and AP-1 are the major transcriptional activators, while NF-IL-6 plays a minor role in IL-8 gene expression (26). Therefore, the activation of IκB kinase or Jun NH2-terminal kinase induced by TRAIL ligation seems to induce IL-8 gene expression. NF-κB has been shown to be activated upon TRAIL ligation (6, 8, 34, 40), although the signal transduction pathway for NF-κB activation has not been elucidated (17, 24). Jun NH2-terminal kinase activation has also been reported to occur upon TRAIL ligation, mediated by either Daxx-ASK-1 (4), RIP/TRAF2 (17, 24), or caspase-dependent pathways (27). Our data indicate that the proteolytic activity of caspases (caspases 1 and 8) is required for the activation of transcription factors such as AP-1 and NF-κB and for subsequent IL-8 promoter activation (Fig. 5C and D). Proteolytic cleavage of kinases and signal transducers by caspases is generally considered an inactivation step occurring during apoptosis (48, 52), however, certain kinases (e.g., MEK kinase 1) are activated after being cleaved by activated caspases (14). Therefore, it is possible that caspases 1 and 8 cleave and activate certain kinases to transduce downstream signals such as those of NF-κB and AP-1. Investigation into this possibility is currently under way.
We have shown that two functional outcomes of DR5 ligation occur in an exclusive manner in both CRT-MG and U87-MG cells (Fig. 3D). These results suggest that IL-8 is not produced as a by-product during the process of dying but rather is actively induced in the live-cell population. Cells in which IL-8 promoter activity is induced upon DR5 ligation are resistant to DR5-mediated apoptosis, and IL-8 promoter activity is not induced in cells undergoing apoptosis. These findings are also true for a single clonal cell line, IL-8p-d2EGFP (Fig. 5E), suggesting that the two independent outcomes upon DR5 ligation are not due to the heterogeneity of the cell lines that we studied. Understanding the molecular mechanisms that determine the fate of cells upon DR5 ligation is important for modulating the biological consequences of the TRAIL-DR5 system in brain tumors and warrants further investigation.
In conclusion, our results suggest that the TRAIL-DR5 system induces a proinflammatory and angiogenic mediator, IL-8, as well as apoptotic cell death in human brain tumors. These results, along with previous findings regarding the proinflammatory properties of the FasL-Fas system, strongly support the notion that members of the TNF/NGF family may regulate tumor growth and homeostasis by either inducing apoptosis or inducing proinflammatory and angiogenic mediators. The proinflammatory role of the TRAIL-DR5 system is not restricted to brain tumor cells. We have demonstrated that human endothelial cells also produce IL-8 upon TRAIL ligation (data not shown). Therefore, TRAIL, FasL, and their receptors, expressed at high levels in human brain tumors and other solid tumors, should also be considered regulators of tumor growth through the induction of angiogenic and growth-promoting mediators or the induction of apoptotic cell death. Since TRAIL is now being studied for immunotherapy in cancer patients, these findings also have implications for the possible beneficial effects of TRAIL combined with antiangiogenesis therapy against IL-8.
Acknowledgments
We thank Naofumi Mukaida (Kanazawa University, Kanazawa, Japan) for the human IL-8 promoter contruct.
This work was supported in part by National Institutes of Health grants MH55795, NS36765, and NS29719 (to E.N.B.). We acknowledge the support of the University of Alabama at Birmingham Flow Cytometry Core Facility (grant AM20614).
REFERENCES
- 1.Addison, C. L., T. O. Daniel, M. D. Burdick, H. Liu, J. E. Ehlert, Y. Y. Xue, L. Buechi, A. Walz, A. Richmond, and R. M. Strieter. 2000. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J. Immunol. 165:5269–5277. [DOI] [PubMed] [Google Scholar]
- 2.Arenberg, D. A., S. L. Kunkel, P. J. Polverini, M. Glass, M. D. Burdick, and R. M. Strieter. 1996. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J. Clin. Investig. 97:2792–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggiolini, M., A. Walz, and S. L. Kunkel. 1989. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 84:1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, H. Y., H. Nishitoh, X. Yang, H. Ichijo, and D. Baltimore. 1998. Activation of apoptosis signal-regulating kinase 1 (ASK1) by the adapter protein Daxx. Science 281:1860–1863. [DOI] [PubMed] [Google Scholar]
- 5.Chang, H. Y., and X. Yang. 2000. Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 64:821–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary, P. M., M. Eby, A. Jasmin, A. Bookwalter, J. Murray, and L. Hood. 1997. Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7:821–830. [DOI] [PubMed] [Google Scholar]
- 7.Choi, C., X. Xu, J. W. Oh, S. J. Lee, G. Y. Gillespie, H. Park, H. Jo, and E. N. Benveniste. 2001. Fas-induced expression of chemokines in human glioma cells: involvement of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase. Cancer Res. 61:3084–3091. [PubMed] [Google Scholar]
- 8.Degli-Esposti, M. A., W. C. Dougall, P. J. Smolak, J. Y. Waugh, C. A. Smith, and R. G. Goodwin. 1997. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7:813–820. [DOI] [PubMed] [Google Scholar]
- 9.Desbaillets, I., A. C. Diserens, N. de Tribolet, M. F. Hamou, and E. G. Van Meir. 1999. Regulation of interleukin-8 expression by reduced oxygen pressure in human glioblastoma. Oncogene 18:1447–1456. [DOI] [PubMed] [Google Scholar]
- 10.Desbaillets, I., A. C. Diserens, N. Tribolet, M. F. Hamou, and E. G. Van Meir. 1997. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. J. Exp. Med. 186:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantuzzi, G., and C. A. Dinarello. 1999. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 19:1–11. [DOI] [PubMed] [Google Scholar]
- 12.Frank, S., U. Kohler, G. Schackert, and H. K. Schackert. 1999. Expression of TRAIL and its receptors in human brain tumors. Biochem. Biophys. Res. Commun. 257:454–459. [DOI] [PubMed] [Google Scholar]
- 13.French, L. E., and J. Tschopp. 1999. The TRAIL to selective tumor death. Nat. Med. 5:146–147. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, S., C. Widmann, and G. L. Johnson. 1999. Differential involvement of MEK kinase 1 (MEKK1) in the induction of apoptosis in response to microtubule-targeted drugs versus DNA damaging agents. J. Biol. Chem. 274:10916–10922. [DOI] [PubMed] [Google Scholar]
- 15.Green, D. R. 1998. Apoptosis. Death deceiver. Nature 396:629–630. [DOI] [PubMed] [Google Scholar]
- 16.Gura, T. 1997. How TRAIL kills cancer cells, but not normal cells. Science 277:768. [DOI] [PubMed] [Google Scholar]
- 17.Hu, W. H., H. Johnson, and H. B. Shu. 1999. Tumor necrosis factor-related apoptosis-inducing ligand receptors signal NF-kappaB and JNK activation and apoptosis through distinct pathways. J. Biol. Chem. 274:30603–30610. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa, K., W. Liu, L. Zhao, Z. Wang, D. Liu, T. Ohtsuka, H. Zhang, J. D. Mountz, W. J. Koopman, R. P. Kimberly, and T. Zhou. 2001. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat. Med. 7:954–960. [DOI] [PubMed] [Google Scholar]
- 19.Inoue, K., J. W. Slaton, B. Y. Eve, S. J. Kim, P. Perrotte, M. D. Balbay, S. Yano, M. Bar-Eli, R. Radinsky, C. A. Pettaway, and C. P. Dinney. 2000. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin. Cancer Res. 6:2104–2119. [PubMed] [Google Scholar]
- 20.Kischkel, F. C., D. A. Lawrence, A. Chuntharapai, P. Schow, K. J. Kim, and A. Ashkenazi. 2000. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12:611–620. [DOI] [PubMed] [Google Scholar]
- 21.Koch, A. E., P. J. Polverini, S. L. Kunkel, L. A. Harlow, L. A. DiPietro, V. M. Elner, S. G. Elner, and R. M. Strieter. 1992. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258:1798–1801. [DOI] [PubMed] [Google Scholar]
- 22.Legler, J. M., L. A. Ries, M. A. Smith, J. L. Warren, E. F. Heineman, R. S. Kaplan, and M. S. Linet. 1999. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J. Natl. Cancer Inst. 91:1382–1390. [DOI] [PubMed] [Google Scholar]
- 23.Li, M., V. O. Ona, C. Guegan, M. Chen, V. Jackson-Lewis, L. J. Andrews, A. J. Olszewski, P. E. Stieg, J. P. Lee, S. Przedborski, and R. M. Friedlander. 2000. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science 288:335–339. [DOI] [PubMed] [Google Scholar]
- 24.Lin, Y., A. Devin, A. Cook, M. M. Keane, M. Kelliher, S. Lipkowitz, and Z. G. Liu. 2000. The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IκB kinase and c-Jun N-terminal kinase. Mol. Cell. Biol. 20:6638–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Los, M., S. Wesselborg, and K. Schulze-Osthoff. 1999. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity 10:629–639. [DOI] [PubMed] [Google Scholar]
- 26.Matsusaka, T., K. Fujikawa, Y. Nishio, N. Mukaida, K. Matsushima, T. Kishimoto, and S. Akira. 1993. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA 90:10193–10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mühlenbeck, F., E. Haas, R. Schwenzer, G. Schubert, M. Grell, C. Smith, P. Scheurich, and H. Wajant. 1998. TRAIL/Apo2L activates c-Jun NH2-terminal kinase (JNK) via caspase-dependent and caspase-independent pathways. J. Biol. Chem. 273:33091–33098. [DOI] [PubMed] [Google Scholar]
- 28.Nagane, M., G. Pan, J. J. Weddle, V. M. Dixit, W. K. Cavenee, and H. J. Huang. 2000. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 60:847–853. [PubMed] [Google Scholar]
- 29.Nagata, S. 1994. Fas and Fas ligand: a death factor and its receptor. Adv. Immunol. 57:129–144. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura, M., J. Rieger, M. Weller, J. Kim, P. Kleihues, and H. Ohgaki. 2000. APO2L/TRAIL expression in human brain tumors. Acta Neuropathol. (Berlin) 99:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen, V. T., W. S. Walker, and E. N. Benveniste. 1998. Post-transcriptional inhibition of CD40 gene expression in microglia by transforming growth factor-beta. Eur. J. Immunol. 28:2537–2548. [DOI] [PubMed] [Google Scholar]
- 32.Oh, J. W., L. M. Schwiebert, and E. N. Benveniste. 1999. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J. Neurovirol. 5:82–94. [DOI] [PubMed] [Google Scholar]
- 33.Ona, V. O., M. Li, J. P. Vonsattel, L. J. Andrews, S. Q. Khan, W. M. Chung, A. S. Frey, A. S. Menon, X. J. Li, P. E. Stieg, J. Yuan, J. B. Penney, A. B. Young, J. H. Cha, and R. M. Friedlander. 1999. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington’s disease. Nature 399:263–267. [DOI] [PubMed] [Google Scholar]
- 34.Pan, G., K. O’Rourke, A. M. Chinnaiyan, R. Gentz, R. Ebner, J. Ni, and V. M. Dixit. 1997. The receptor for the cytotoxic ligand TRAIL. Science 276:111–113. [DOI] [PubMed] [Google Scholar]
- 35.Rieger, J., U. Naumann, T. Glaser, A. Ashkenazi, and M. Weller. 1998. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 427:124–128. [DOI] [PubMed] [Google Scholar]
- 36.Rieger, J., H. Ohgaki, P. Kleihues, and M. Weller. 1999. Human astrocytic brain tumors express AP02L/TRAIL. Acta Neuropathol. (Berlin) 97:1–4. [DOI] [PubMed] [Google Scholar]
- 37.Roth, W., S. Isenmann, U. Naumann, S. Kugler, M. Bahr, J. Dichgans, A. Ashkenazi, and M. Weller. 1999. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem. Biophys. Res. Commun. 265:479–483. [DOI] [PubMed] [Google Scholar]
- 38.Saas, P., J. Boucraut, A. L. Quiquerez, V. Schnuriger, G. Perrin, S. Desplat-Jego, D. Bernard, P. R. Walker, and P. Y. Dietrich. 1999. CD95 (Fas/Apo-1) as a receptor governing astrocyte apoptotic or inflammatory responses: a key role in brain inflammation? J. Immunol. 162:2326–2333. [PubMed] [Google Scholar]
- 39.Salcedo, R., J. H. Resau, D. Halverson, E. A. Hudson, M. Dambach, D. Powell, K. Wasserman, and J. J. Oppenheim. 2000. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. FASEB J. 14:2055–2064. [DOI] [PubMed] [Google Scholar]
- 40.Schneider, P., M. Thome, K. Burns, J. L. Bodmer, K. Hofmann, T. Kataoka, N. Holler, and J. Tschopp. 1997. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity 7:831–836. [DOI] [PubMed] [Google Scholar]
- 41.Seol, D. W., and T. R. Billiar. 1999. A caspase-9 variant missing the catalytic site is an endogenous inhibitor of apoptosis. J. Biol. Chem. 274:2072–2076. [DOI] [PubMed] [Google Scholar]
- 42.Singh, R. K., and M. L. Varney. 2000. IL-8 expression in malignant melanoma: implications in growth and metastasis. Histol. Histopathol. 15:843–849. [DOI] [PubMed] [Google Scholar]
- 43.Smith, D. R., P. J. Polverini, S. L. Kunkel, M. B. Orringer, R. I. Whyte, M. D. Burdick, C. A. Wilke, and R. M. Strieter. 1994. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J. Exp. Med. 179:1409–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sprick, M. R., M. A. Weigand, E. Rieser, C. T. Rauch, P. Juo, J. Blenis, P. H. Krammer, and H. Walczak. 2000. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12:599–609. [DOI] [PubMed] [Google Scholar]
- 45.Strieter, R. M., P. J. Polverini, D. A. Arenberg, and S. L. Kunkel. 1995. The role of CXC chemokines as regulators of angiogenesis. Shock 4:155–160. [DOI] [PubMed] [Google Scholar]
- 46.Strieter, R. M., P. J. Polverini, D. A. Arenberg, A. Walz, G. Opdenakker, J. Van Damme, and S. L. Kunkel. 1995. Role of C-X-C chemokines as regulators of angiogenesis in lung cancer. J. Leukoc. Biol. 57:752–762. [DOI] [PubMed] [Google Scholar]
- 47.Strieter, R. M., P. J. Polverini, S. L. Kunkel, D. A. Arenberg, M. D. Burdick, J. Kasper, J. Dzuiba, J. Van Damme, A. Walz, D. Marriott, et al. 1995. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 270:27348–27357. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki, K., T. Hasegawa, C. Sakamoto, Y. M. Zhou, F. Hato, M. Hino, N. Tatsumi, and S. Kitagawa. 2001. Cleavage of mitogen-activated protein kinases in human neutrophils undergoing apoptosis: role in decreased responsiveness to inflammatory cytokines. J. Immunol. 166:1185–1192. [DOI] [PubMed] [Google Scholar]
- 49.Tatsuta, T., A. Shiraishi, and J. D. Mountz. 2000. The prodomain of caspase-1 enhances Fas-mediated apoptosis through facilitation of caspase-8 activation. J. Biol. Chem. 275:14248–14254. [DOI] [PubMed] [Google Scholar]
- 50.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312–1316. [DOI] [PubMed] [Google Scholar]
- 51.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Kovalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17:331–367. [DOI] [PubMed] [Google Scholar]
- 52.Widmann, C., S. Gibson, and G. L. Johnson. 1998. Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J. Biol. Chem. 273:7141–7147. [DOI] [PubMed] [Google Scholar]
- 53.Wu, M., A. Das, Y. Tan, C. Zhu, T. Cui, and M. C. Wong. 2000. Induction of apoptosis in glioma cell lines by TRAIL/Apo-2L. J. Neurosci. Res. 61:464–470. [DOI] [PubMed] [Google Scholar]
- 54.Zamai, L., M. Ahmad, I. M. Bennett, L. Azzoni, E. S. Alnemri, and B. Perussia. 1998. Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 188:2375–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]