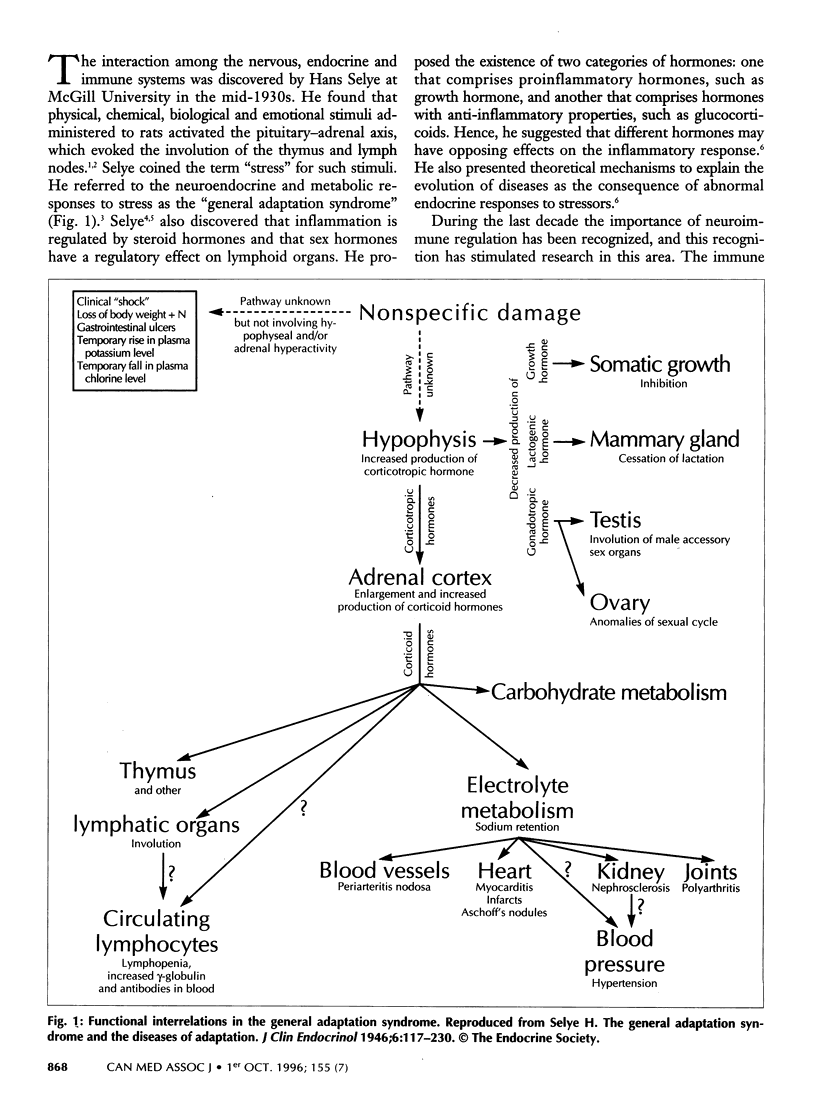
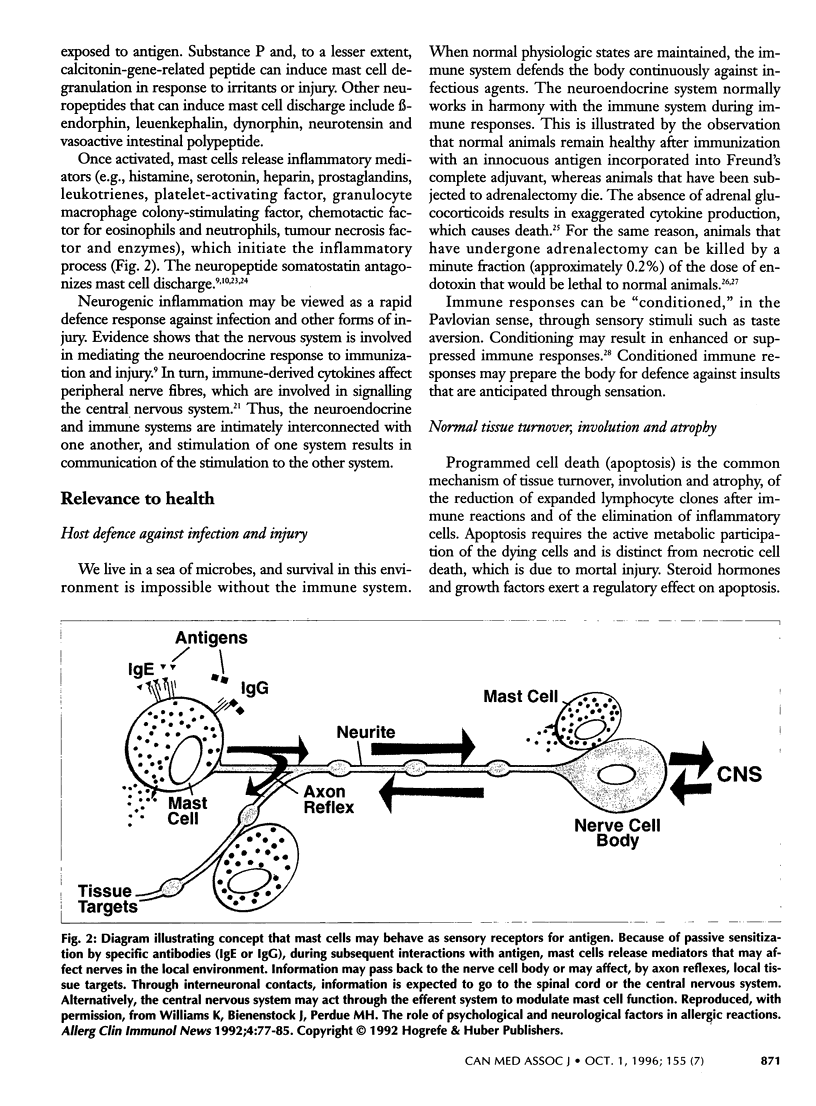
Abstract
A novel scientific discipline that examines the complex interdependence of the neural, endocrine and immune systems in health and disease has emerged in recent years. In health, the neuroimmunoregulatory network is fundamental to host defence and to the transfer of immunity to offspring; the network also plays important roles in intestinal physiology and in tissue regeneration, healing and reproduction. The proliferation of lymphocytes in primary lymphoid organs (bone marrow, bursa of Fabricius [in birds] and thymus) and in secondary lymphoid organs (spleen, lymph nodes and mucosal lymphoid tissue) depends on prolactin and growth hormone. These hormones allow immune cells to respond to antigen and to soluble mediators, called cytokines. Immune-derived cytokines are capable of inducing fever and of altering neuro-transmitter activity in the brain and hormone secretion by the pituitary gland. The activation of the hypothalamus-pituitary-adrenal axis by cytokines leads to immunosuppression. Lymphoid organs are innervated, and tissue mast cells respond to neurologic stimuli. In general, acetylcholine and substance P exert immunostimulatory and proinflammatory effects, whereas epinephrine and somatostatin are immunosuppressive and anti-inflammatory. In this article, the authors predict that novel approaches to immunomodulation will be possible by altering the level or efficacy of immunoregulatory hormones and neurotransmitters.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi M. The maternal-fetal interaction: some controversies and solutions. Exp Clin Immunogenet. 1993;10(2):103–117. [PubMed] [Google Scholar]
- Anisman H., Zalcman S., Zacharko R. M. The impact of stressors on immune and central neurotransmitter activity: bidirectional communication. Rev Neurosci. 1993 Apr-Jun;4(2):147–180. doi: 10.1515/revneuro.1993.4.2.147. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H. Apoptosis: mechanisms and roles in pathology. Int Rev Exp Pathol. 1991;32:223–254. doi: 10.1016/b978-0-12-364932-4.50010-1. [DOI] [PubMed] [Google Scholar]
- Berczi I., Baragar F. D., Chalmers I. M., Keystone E. C., Nagy E., Warrington R. J. Hormones in self tolerance and autoimmunity: a role in the pathogenesis of rheumatoid arthritis? Autoimmunity. 1993;16(1):45–56. doi: 10.3109/08916939309010647. [DOI] [PubMed] [Google Scholar]
- Berczi I., Chalmers I. M., Nagy E., Warrington R. J. The immune effects of neuropeptides. Baillieres Clin Rheumatol. 1996 May;10(2):227–257. doi: 10.1016/s0950-3579(96)80016-1. [DOI] [PubMed] [Google Scholar]
- Berczi I. The role of the growth and lactogenic hormone family in immune function. Neuroimmunomodulation. 1994 Jul-Aug;1(4):201–216. doi: 10.1159/000097168. [DOI] [PubMed] [Google Scholar]
- Carlson S. L., Felten D. L., Livnat S., Felten S. Y. Alterations of monoamines in specific central autonomic nuclei following immunization in mice. Brain Behav Immun. 1987 Mar;1(1):52–63. doi: 10.1016/0889-1591(87)90006-7. [DOI] [PubMed] [Google Scholar]
- Kabiersch A., del Rey A., Honegger C. G., Besedovsky H. O. Interleukin-1 induces changes in norepinephrine metabolism in the rat brain. Brain Behav Immun. 1988 Sep;2(3):267–274. doi: 10.1016/0889-1591(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Klein T. W., Newton C., Friedman H. Cannabinoids and immunity to Legionella pneumophila infection. Adv Exp Med Biol. 1996;402:103–109. doi: 10.1007/978-1-4613-0407-4_15. [DOI] [PubMed] [Google Scholar]
- McCann S. M., Karanth S., Kamat A., Dees W. L., Lyson K., Gimeno M., Rettori V. Induction by cytokines of the pattern of pituitary hormone secretion in infection. Neuroimmunomodulation. 1994 Jan;1(1):2–13. doi: 10.1159/000095948. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., McKay D. M. Integrative immunophysiology in the intestinal mucosa. Am J Physiol. 1994 Aug;267(2 Pt 1):G151–G165. doi: 10.1152/ajpgi.1994.267.2.G151. [DOI] [PubMed] [Google Scholar]
- Perretti M., Mugridge K. G., Becherucci C., Parente L. Evidence that interleukin-1 and lipoxygenase metabolites mediate the lethal effect of complete Freund's adjuvant in adrenalectomized rats. Lymphokine Cytokine Res. 1991 Aug;10(4):239–243. [PubMed] [Google Scholar]
- Ramachandra R. N., Sehon A. H., Berczi I. Neuro-hormonal host defence in endotoxin shock. Brain Behav Immun. 1992 Jun;6(2):157–169. doi: 10.1016/0889-1591(92)90015-g. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Seaman M. S., Miller R. E., Tough T. W., Alderson M. R., Lynch D. H. gld/gld mice are unable to express a functional ligand for Fas. Eur J Immunol. 1994 Apr;24(4):928–933. doi: 10.1002/eji.1830240422. [DOI] [PubMed] [Google Scholar]
- SELYE H. Effect of ACTH and cortisone upon an anaphylactoid reaction. Can Med Assoc J. 1949 Dec;61(6):553-6, illust. [PMC free article] [PubMed] [Google Scholar]
- SELYE H. Stress and disease. Science. 1955 Oct 7;122(3171):625–631. doi: 10.1126/science.122.3171.625. [DOI] [PubMed] [Google Scholar]
- Sabbadini E., Berczi I. The submandibular gland: a key organ in the neuro-immuno-regulatory network? Neuroimmunomodulation. 1995 Jul-Aug;2(4):184–202. doi: 10.1159/000097197. [DOI] [PubMed] [Google Scholar]
- South S. A., Yankov V. I., Evans W. S. Normal reproductive neuroendocrinology in the female. Endocrinol Metab Clin North Am. 1993 Mar;22(1):1–28. [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Pekarek R. S., Thompson W. L., Curnow R. T., Beall F. A., Zenser T. V., DeRubertis F. R., Beisel W. R. A protein from polymorphonuclear leukocytes (LEM) which affects the rate of hepatic amino acid transport and synthesis of acute-phase globulins. Endocrinology. 1975 Mar;96(3):651–661. doi: 10.1210/endo-96-3-651. [DOI] [PubMed] [Google Scholar]
- Zalcman S., Green-Johnson J. M., Murray L., Nance D. M., Dyck D., Anisman H., Greenberg A. H. Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res. 1994 Apr 18;643(1-2):40–49. doi: 10.1016/0006-8993(94)90006-x. [DOI] [PubMed] [Google Scholar]


