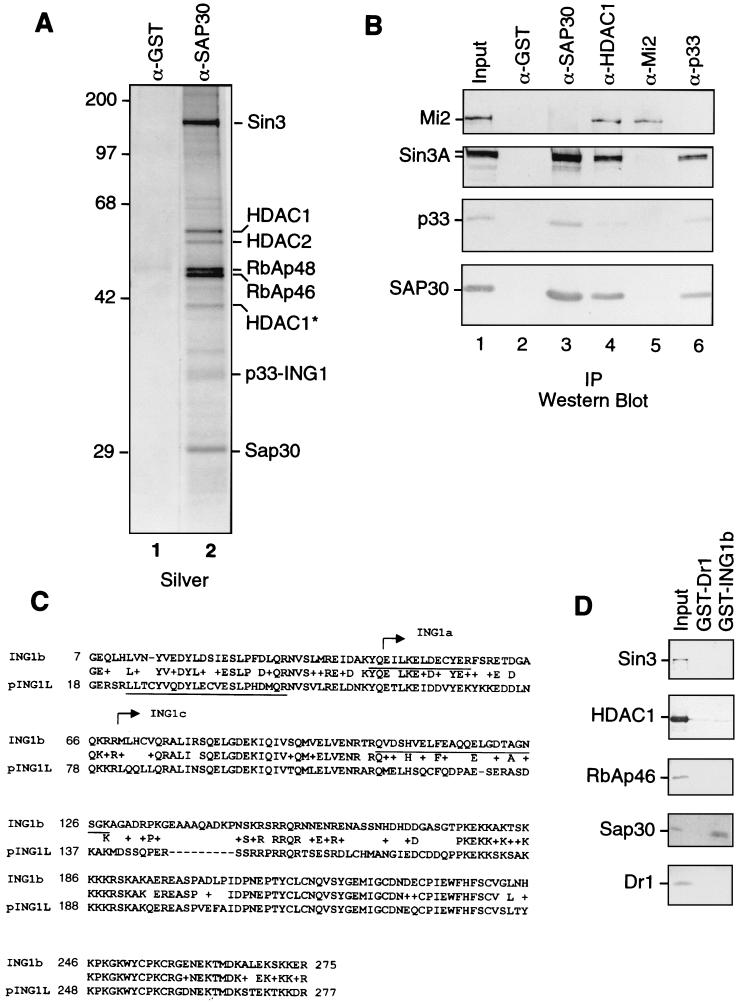
FIG. 1.
Identification of p33ING1b as a SAP30-associated protein. (A) Silver staining of anti-SAP30 immunoprecipitates. An aliquot of the DEAE-52 bound material (∼100 μg) was immunoprecipitated with anti-SAP30 antibodies, and immunoprecipitated proteins were washed with buffer containing 0.5 M KCl and 0.05% NP-40. Proteins were eluted with 0.1 M glycine (pH 2.6) and separated by SDS-PAGE followed by silver staining. (B) Western blots of anti-SAP30, anti-HDAC1, anti-Mi2, and anti-p33 immunoprecipitates. Immunoprecipitations (IP) were performed as in panel A. Input, 10 μg of the DEAE-52-bound fraction. Lanes 2 to 6 correspond to 1/10 of the total glycine eluate from the corresponding affinity columns. (C) Sequence alignment of p33ING1b and pING1L. Peptides derived from the 33-kDa band in anti-SAP30 immunoprecipitates are underlined. The sequences of p33ING1b shared with other ING1 isoforms (ING1a and ING1c) are indicated by arrows. (D) Direct interaction between p33 and SAP30. GST-p33 or GST-Dr1 proteins (100 ng) were attached to glutathione-Sepharose beads (5 μl) and incubated with 200 ng of recombinant purified HDAC1, RbAp46, SAP30, Dr1, or in vitro-translated Sin3 protein in buffer containing 0.5 M KCl, 0.1% NP-40, and 0.1 mg of BSA per ml. The bound proteins were washed with the same buffer and eluted with SDS loading buffer. A 1/10 aliquot of material bound to the beads was analyzed by Western blotting as indicated in the figure. Input, 20 ng of recombinant protein.