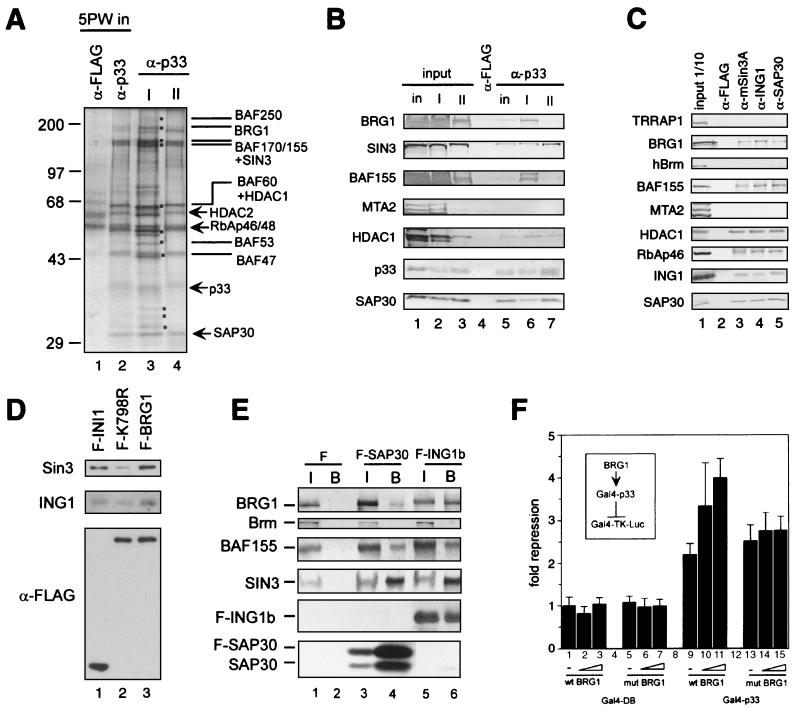
FIG. 4.
Interaction between the Sin3 complex I and the Brg1-based Swi-Snf complex. (A) Silver staining of anti-p33 immunoprecipitates derived from the DEAE-5PW fractions corresponding to Sin3 complexes I and II. Equal p33 Western blot units of DEAE-5PW fractions corresponding to Sin3 complexes I and II or DEAE-5PW input (∼100 μg) were immunoprecipitated with anti-p33 or anti-FLAG antibodies, washed with buffer containing 0.5 M KCl and 0.05% NP-40, and eluted with 0.1 M glycine (pH 2.6). Immunoprecipitated proteins (one-quarter of the total glycine eluate) were resolved by SDS-PAGE and visualized by silver staining. Polypeptides specific for complex I are marked by dots. The subunits of the Sin3 and Swi-Snf complexes are indicated. (B) Western blot of anti-p33 immunoprecipitates from DEAE-5PW input and DEAE-5PW fractions corresponding to Sin3 complexes I and II. Anti-p33 immunoprecipitation from DEAE-5PW input (in) and DEAE-5PW fractions corresponding to Sin3 complexes I (I) and II (II) was performed as described in the legend to panel A, followed by Western blot analysis using different antibodies described on the left side of the panel. Input lanes correspond to 10% of the material used for immunoprecipitation. Lanes corresponding to α-p33 immunoprecipitates contain one-quarter of the total glycine eluates. As a negative control, anti-FLAG immunoprecipitation was performed from the DEAE-5PW input material (column labeled α-FLAG). (C) Western blots of anti-Sin3, anti-ING1b, and SAP30 immunoprecipitates from HeLa nuclear extract. Approximately 300 μg of nuclear extracts was immunoprecipitated with antibodies, as indicated at the top of the panel. Samples were washed and proteins were eluted as described in the legend to panel A. Half of the total glycine eluate was separated by SDS-PAGE and analyzed by Western blotting using the antibodies indicated on the left side of the panel. (D) Sin3 and p33ING1b are associated with the Brg1-based Swi-Snf complex. Nuclear extracts from HeLa cells stably expressing FLAG-tagged wild-type Brg1 (F-BRG1) and ATPase-deficient mutant (K798R) (F-K798R) and FLAG-tagged INI1 (F-INI1) were fractionated on an anti-FLAG agarose column, and bound proteins were eluted with excess FLAG peptide and analyzed by Western blotting as described previously (42). To ensure equal efficiency of immunoprecipitation of FLAG-tagged INI1, Brg1, and Brg1(K798R), the same immunoprecipitates were analyzed by Western blotting with anti-FLAG antibodies (bottom panel). Cell lines have been described previously (42, 43). (E) Endogenous Brg1 and BAF155 but not Brm coimmunoprecipitate with transiently overexpressed FLAG-tagged SAP30 and p33ING1b. Nuclear extracts were prepared from 5 × 107 293T cells transfected with empty vector (F), FLAG-SAP30 (F-SAP30), or FLAG-p33ING1b (F-ING1b) expression vectors. These extracts (300 μg) were immunoprecipitated with anti-FLAG antibodies, and bound proteins were washed with buffer containing 0.5 M KCl and 0.05% NP-40 and eluted with the SDS loading buffer followed by Western blot analysis using antibodies described on the left side of the panel. Input (I) corresponds to 1/10 of the nuclear extract used for immunoprecipitation, whereas the α-FLAG bead-bound fraction (B) corresponds to 1/2 of the material eluted from the beads. (F) Brg1 enhances repression by Gal4-p33ING1b. 293T cells (106 cells per 6-cm plate) were transfected with a luciferase reporter driven by a promoter containing five copies of the Gal4 DNA binding site (Gal-TK-Luc; 250 ng), together with a Gal4-p33 fusion protein (Gal4-p33; 500 ng) or Gal4 DNA-binding domain alone (Gal4-DB; 500 ng) in the presence or absence of the Brg1 expression vector (wtBRG1, or mutBRG1; 250, 500, or 1,000 ng). Luciferase activity was measured as described in Materials and Methods. Fold repression was normalized to reporter activity in the absence of Gal4-p33.