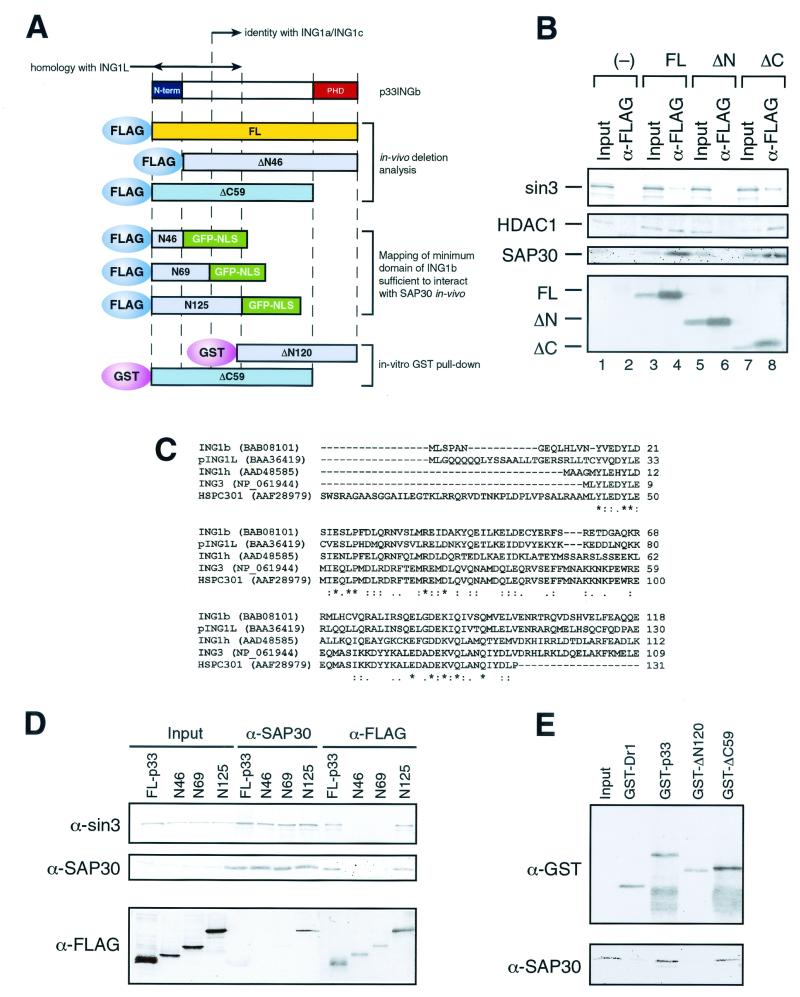
FIG. 5.
The unique N-terminal domain of p33ING1b is required for interaction with the Sin3 complex in vivo. (A) Scheme of ING1 deletion mutants. (B) Western blot analysis of anti-FLAG immunoprecipitates from nuclear extracts of NIH 3T3 cells transfected with FLAG-tagged p33ING1b deletion mutants. Nuclear extracts made from 5 × 107 NIH 3T3 cells transfected with empty vector (−), full-length p33 (FL), and p33 deletion mutants (ΔN and ΔC) were immunoprecipitated with anti-FLAG antibodies. Immunoprecipitated proteins were washed with buffer containing 0.5 M KCl and 0.05% NP-40, eluted with SDS loading buffer, and analyzed by Western blotting using antibodies specific for Sin3,HDAC1, and SAP30. Input corresponds to 1/10 of the nuclear extract used for immunoprecipitation, and α-FLAG corresponds to 1/2 of the eluate from the α-FLAG beads. The bottom panel shows expression of the transfected p33ING1b deletion mutants detected using anti FLAG-antibodies. (C) Sequence alignment of proteins containing the domain similar to the N terminus of p33ING1. Alignment was done using the CLUSTALW program at the European Bioinformatics Institute server (http://www2.ebi.ac.uk/clustalw) (48). Asterisks indicate identical positions, colons indicate conserved substitutions, and dots indicates semiconserved (i.e., conserved in most but not all of the aligned sequences) substitutions. (D) Identification of a minimum domain of p33ING1b sufficient to interact with the Sin3 complex in vivo. 239T cells were transfected with either full-length FLAG-tagged p33 protein (FL-p33) or FLAG-tagged fusion proteins containing the N-terminal 46, 69, or 125 amino acids of the p33 protein fused with GFP (N46, N69, and N125). Nuclear extracts from transfected cells (∼1 mg) were immunoprecipitated either with anti-SAP30 or with anti-FLAG antibody, and aliquots of immunoprecipitated proteins (1/2) were analyzed by Western blotting as indicated on the left side of the panel. Inputs correspond to 5 μg (1/200) of nuclear extracts used for immunoprecipitation. (E) The N terminus of p33 is required for the interaction with SAP30 in vitro. GST pull-down was performed as described in the legend to Fig. 1D, using 100 ng of the indicated GST fusion protein and 100 ng of recombinant SAP30. Aliquots of the reactions (1/2) were analyzed by Western blotting using anti-GST and anti-SAP30 antibodies. Input corresponds to 10 ng of recombinant SAP30.