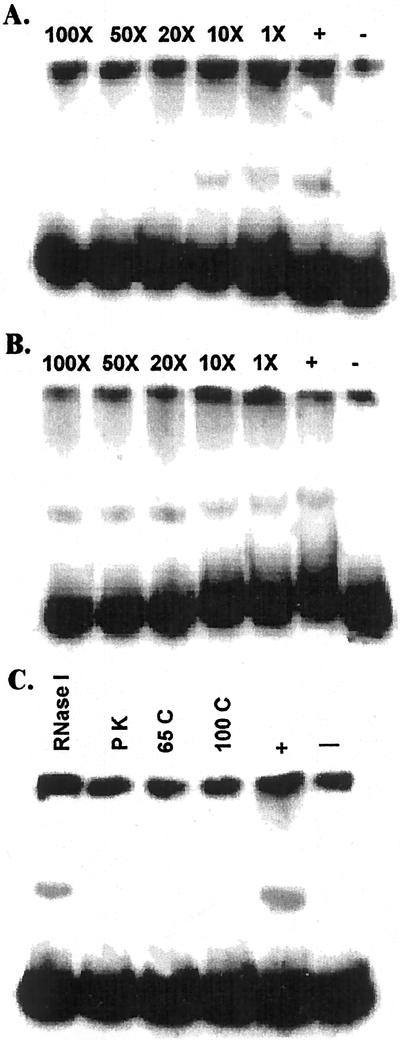
Figure 6.
Nuclear proteins interact specifically with promoter fragments. A, Labeled DNA fragment IV of TPSI1 promoter was mixed with nuclear extracts obtained from Pi-starved (−) or -sufficient (+) Arabidopsis tissue. Indicated mass excess of unlabeled fragment IV was added as specific competitors. Increasing concentrations of the competitor progressively reduced the binding of the labeled fragment. B, Increasing quantities of pBluescript DNA digested with HhaI and Sau3A were added to the DNA (TPSI1 promoter fragment IV)-protein interaction mix. Lack of competition with increasing mass excess of non-specific competitor indicated that the interaction between the DNA and the nuclear proteins is specific. C, The DNA binding factors are proteinaceous in nature. Nuclear proteins from Pi-sufficient tomato roots were subject to RNaseI and ProteinaseK (PK) treatments and heat denaturation (65°C and 100°C) for 10 min. The treated nuclear proteins were used in gel retardation reactions with the TPSI1 promoter fragment IV. The treatments that denature proteins resulted in a loss of DNA-protein interaction, whereas RNaseI treatment did not affect binding.