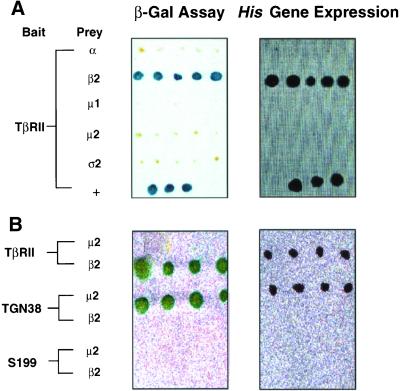
Figure 6.
Cytoplasmic domain of TβRII interacts with the β2 subunit of AP2. Yeast strain Y190 was transformed with bait plasmid consisting of the cytoplasmic domain of TβRII, the S199 TβRII mutant, or the TGN38 cytoplasmic tail fused downstream of the DNA binding domain of GAL4 (GAL4BD). Clones expressing bait proteins were selected on Trp− plates and transformed with the indicated AP subunits fused downstream of the activation domain of GAL4 (GAL4AD). Double transformants were tested for reporter gene expression. (A) Left, double transformants capable of growth on Trp−Leu− plates were lysed and β-galactosidase expression was determined by incubating with 80 μg/ml X-gal (Invitrogen) at 37°C for 2–6 h; blue colonies indicate protein interactions. Right, clones assessed in the left panel were streaked on Trp−Leu−His− plates; growth indicates protein interactions. Y190 cotransformed with GAL4BD-p53 and GAL4AD-TAg (large T antigen) was used as positive controls (+). (B) Studies similar to those described in A were performed using the indicated proteins as bait and prey.