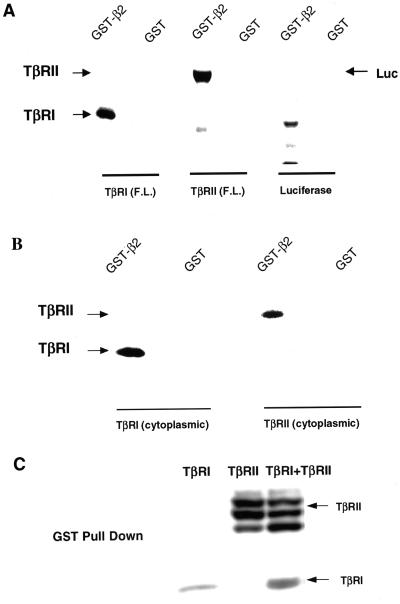
Figure 7.
Type I and type II TGF-β receptors bind the β2 subunit of AP2. (A) Full-length (F.L.) TβRI, TβRII or luciferase were in vitro translated in the presence of [35S]methionine. Equal molar amounts were suspended in 400 μl of binding buffer (see MATERIALS AND METHODS). Then 10 μg of GST alone or GST-β2 bound to glutathione-agarose beads was added and the mixture rocked 1 h at 22°C. After extensive washing, bound material was analyzed by SDS-PAGE and autoradiography (see MATERIALS AND METHODS). Analogous results were obtained when binding was performed in reticulocyte lysate (our unpublished data). (B) Studies identical to those described in A were performed using in vitro translated cytosolic domains of TβRI or TβRII in 400 μl of rabbit reticulocyte lysate supplemented with an ATP-regenerating system. Similar results (our unpublished data) were obtained when the binding was carried out in binding buffer as in A. (C) Cos7 cells were transiently transfected with HA-tagged TβRI and/or TβRII. After 24 h, cells were lysed as described under GST fusion protein binding (see MATERIALS AND METHODS). Lysates were normalized for equal receptor expression and incubated with 10 μg of GST-β2. After washing, bound material was separated by SDS-PAGE and analyzed by immunoblotting with α-HA. Each panel depicts a representative experiment of five.