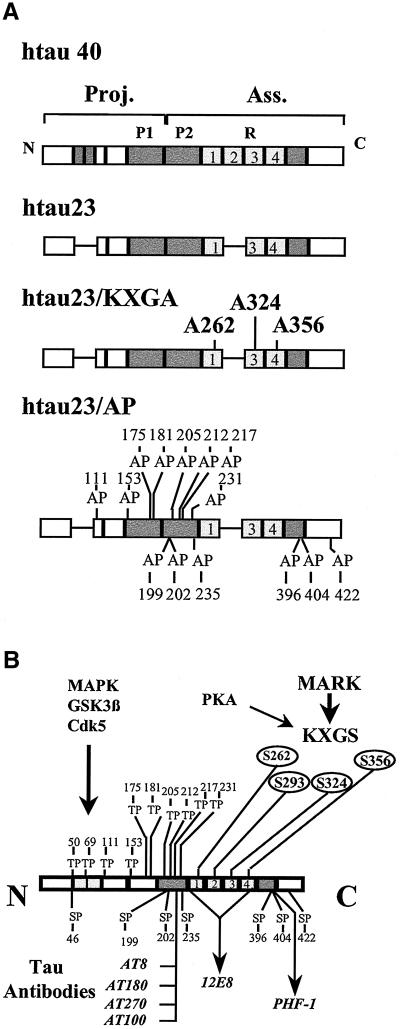
Figure 1.
Bar diagram of human tau, phosphorylation sites, and antibody epitopes. (a) N-terminal half is the “projection domain” because it projects from the microtubule surface but does not bind itself. The C-terminal half is the “assembly domain” because it binds to microtubules and promotes their assembly. The binding is mediated by the three to four pseudorepeats (∼31 residues each) and the flanking domains (shaded). Compared with the longest isoform in the CNS (htau40, 441 residues), the fetal isoform htau23 (352 residues) lacks the second repeat (exon 10) and two inserts near the N terminus (58 residues). The SP or TP motifs (outside the repeat domain, 14 in htau23, 17 in htau40) are marked; in the AP-htau23 mutants the S or T residues are replaced by A so that they cannot be phosphorylated by proline-directed kinases. The KXGS motifs (one per repeat) are the targets of MARK; in the KXGA-htau23 mutant the S residues are replaced by A, making them nonposphorylatable. (b) Antibody epitopes in htau40 sensitive to phosphorylation are indicated. The main targets of cdk5 or cdc2 on htau40 are the double motifs T231/S235 (epitope of antibody AT-180), S202/T205 (antibody AT-8), and S404 (only weak reaction with PHF-1); the main targets of GSK-3β are S396/S404 (strong reaction with antibody PHF-1), and S202/T205 (AT-8 epitope) (Illenberger et al., 1998); the main target of MARK is S262 (epitope of antibody 12E8).