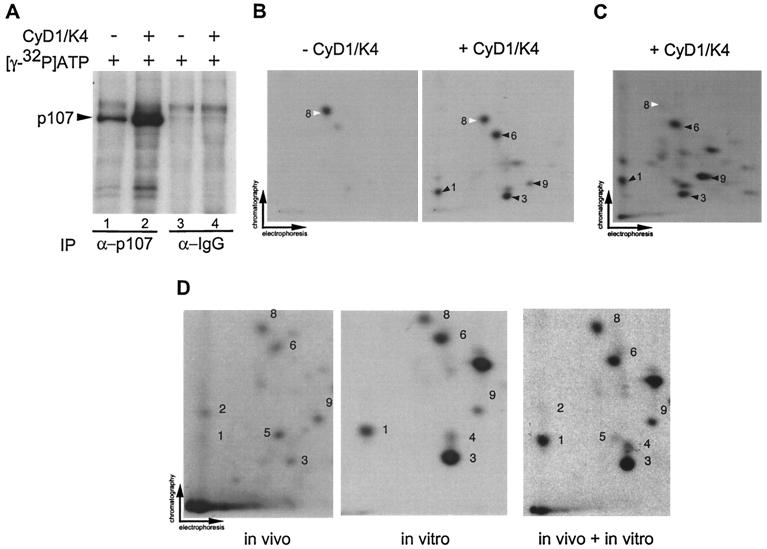
FIG. 5.
Visualization of cyclin D1/Cdk4-specific phosphorylation events in p107. (A) Anti-p107 or control immune complexes from asynchronous C33A cells were incubated with [γ-32P]ATP in the presence or absence of purified cyclin D1/Cdk4 (40 nM). Reaction mixtures were separated by SDS-8% PAGE and transferred to nitrocellulose prior to autoradiography. (B) Two-dimensional tryptic phosphopeptide mapping of samples derived from panel A. In the absence of Cdk4, the single major phosphopeptide 8 was found (white arrow). In the presence of Cdk4, four additional phosphopeptides (peptides 1, 3, 6, and 9) were observed (black arrow). (C) Phosphorylation of p107 immune complexes from Saos-2 cells by cyclin D1/Cdk4 in vitro. pcDNA-p107HA was transiently expressed in Saos-2 cells, and anti-HA immune complexes were then used in kinase assays with cyclin D1/Cdk4 as described in panel B. Phosphorylated p107HA was subjected to tryptic peptide mapping. The major phosphopeptides seen with Cdk4 in panel B were found in panel C (peptides 1, 3, 6, and 9) as indicated by the black arrows. Peptide 8 (white arrow) was weakly phosphorylated in this setting. (D) In vivo phosphorylation of p107. p107HA was expressed in U2OS cells by transient transfection prior to metabolic labeling and analysis as described in Materials and Methods. Labeled p107 (600 cpm) was subjected to peptide mapping in the presence or absence of in vitro-phosphorylated p107HA obtained by immunoprecipitation from U2OS cells.