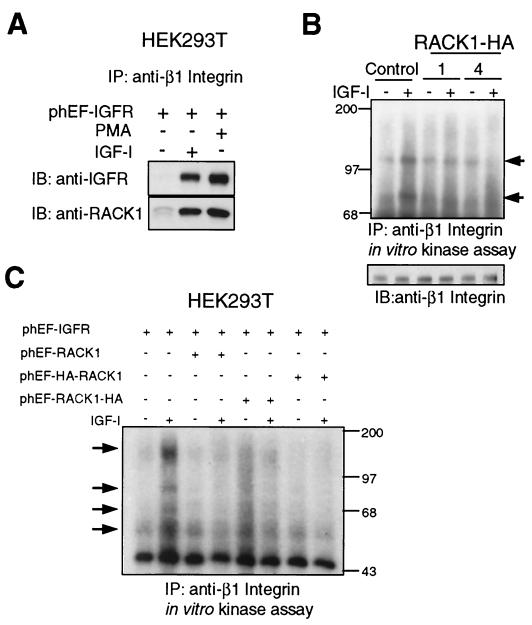
FIG. 7.
IGF-I and PMA induce the association of IGF-IR, RACK1, and β1 integrin, and RACK1 overexpression inhibits IGF-I-dependent β1 integrin-associated kinase activity. (A) HEK293T cells were transiently transfected with phEF-IGFR, serum starved, and either mock treated or treated with IGF-I or PMA for 10 min as described in Materials and Methods. Cell lysates were prepared with digitonin buffer, and 1 mg of total lysate was immunoprecipitated (IP) with anti-β1 integrin Ab. Immunoprecipitates were fractionated in an SDS-12% polyacrylamide gel and transferred to a nitrocellulose membrane, which was cut in half and immunoblotted (IB) with anti-IGFR (upper half) or anti-RACK1 (lower half) Ab. (B) Control and RACK1-overexpressing (clone 1 and 4) NIH 3T3-IGFR cells were serum starved overnight and treated with IGF-I for 10 min. Cell lysates were prepared with CHAPS buffer, and 500 μg of total lysate was immunoprecipitated with anti-β1 integrin Ab and analyzed for in vitro kinase activity as described in Materials and Methods. Arrows indicate the proteins phosphorylated in vitro (upper panel). Equivalent amounts of lysates were analyzed directly by fractionation in SDS-10% polyacrylamide gels and immunoblotting with anti-β1 integrin Ab (lower panel). (C) HEK293T cells were transiently cotransfected with the indicated plasmids as detailed in Materials and Methods, serum starved, and treated with IGF-I as described for panel B. Arrows indicate the proteins phosphorylated in vitro.