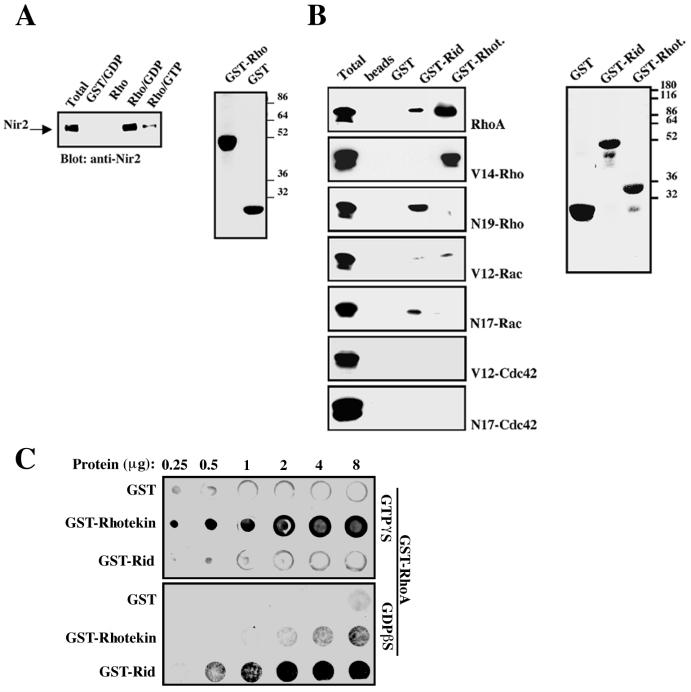
FIG. 6.
Nir2 preferentially binds the inactive form of RhoA via its Rid. (A) In vitro binding of Nir2 to GDP-bound GST-RhoA. Bacterially expressed GST or GST-RhoA fusion proteins were purified on glutathione-agarose beads. The GST-RhoA fusion protein was either preloaded with GDP or GTPγS or left free of nucleotides. The purified recombinant proteins were then incubated with cell lysate of HEK 293 cells expressing the wild-type Nir2, and binding of Nir2 was detected by Western blotting. (B) In vitro binding of Rho-family small GTPases to recombinant Rid. Rid and the RBD of rhotekin (GST-Rhot.) (32) were expressed as GST fusion proteins in bacteria. The recombinant proteins were purified on glutathione-agarose beads and incubated with cell lysate of HEK 293 cells expressing the indicated small GTPase. The same binding assays were carried out using glutathione beads or recombinant GST as a control. Binding of the small GTPases was determined by Western blotting with the appropriate antibodies. The purified GST fusion proteins used in these binding experiments were resolved on an SDS-15% polyacrylamide gel and stained with Coomassie brilliant blue (right panels in panels A and B). Numbers at the extreme right of panels A and B show molecular mass in kilodaltons. (C) Direct binding of Rho to Rid with an overlay assay. Equal amounts of purified GST, GST-rhotekin, and GST-Rid recombinant proteins (0.25 to 8 μg) were loaded on a nitrocellulose membrane and probed with GST-Rho bound to either GDP or GTP as described in Materials and Methods. Following washing, the membranes were probed with anti-Rho antibody and then with horseradish peroxidase-conjugated anti-mouse IgG. The bound Rho was detected by ECL.