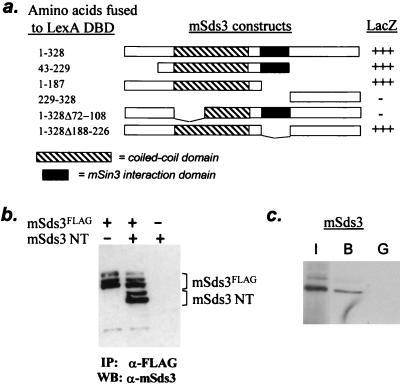
FIG. 4.
mSds3 forms homodimers. (a) Yeast two-hybrid studies using various mSds3 baits along with VP16 TAD-full-length mSds3 prey to localize mSds3's dimerization domain. Helix A of the putative coiled-coil domain (residues 72 to 108) was defined by secondary structure prediction software. (b) In vivo expression of mSds3FLAG along with mSds3 N-terminal fragment (mSds3NT: residues 1 to 187) in 293T cells followed by immunoprecipitation with anti-FLAG antibody results in coimmunoprecipitation of mSds3NT detected by Western blot using anti-mSds3 antibody. IP, immunoprecipitation; WB, Western blot. (c) Bacterially expressed GST-full-length mSds3 fusion protein was tested for its ability to interact with radiolabeled in vitro translated full-length mSds3. I, input, 10%; B, bound (GST-mSds3); G, GST alone.