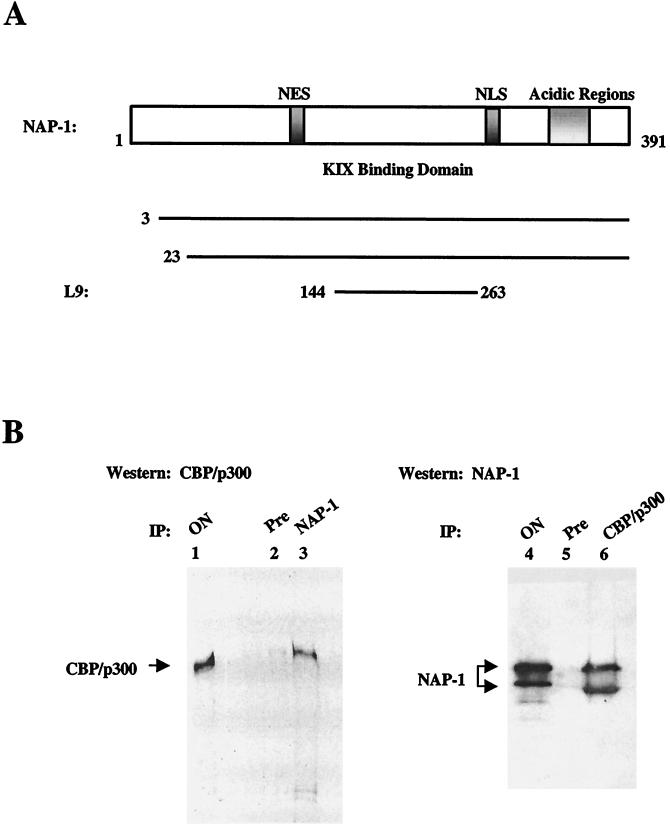
FIG. 1.
NAP-1 interacts with p300 in vivo. (A) The top portion shows the structure of mammalian NAP-1 acidic regions that mediate histone H2A-H2B binding in hatched NAP-1. Consensus nuclear export (NES; aa 78 to 86) and nuclear import (NLS; aa 307 to 327) signals are indicated. The KIX binding domain (aa 144 to 263) is shown. Amino acid endpoints of independent human NAP-1 cDNA clones identified in two-hybrid screen are shown. (B) Coimmunoprecipitation studies of NAP-1 and p300 with whole-cell extracts prepared from HeLa cells. The left panel shows a Western blot assay of p300 recovered from immunoprecipitates prepared with preimmune (Pre; lane 2) or NAP-1 (lane 3) antiserum. The right panel shows a Western blot assay of NAP-1 recovered from immunoprecipitates prepared with preimmune (lane 5) or p300 (lane 6) antiserum. ON, onput protein from HeLa whole cell extract. IP, immunoprecipitate.