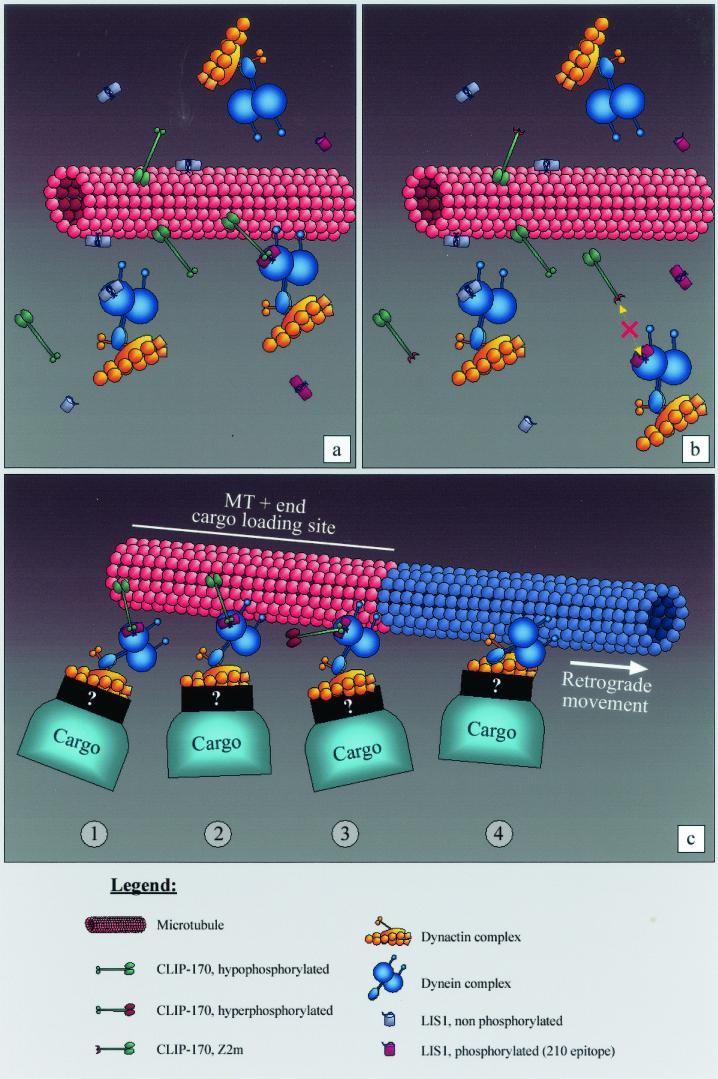
FIG. 7.
A model of LIS1 mediating CLIP-170 interactions with the dynein/dynactin pathway. (a) LIS1 can bind to MT bundles; however, phospho-LIS1 (recognized by the 210 antibody) binding to MT bundles is mediated through the distal zinc finger motif of CLIP-170. (b) When the distal zinc finger motif of CLIP-170 is mutated, the phospho-LIS1 isoform can no longer bind to MT bundles. This can also explain why p150/dynactin is not recruited to the MT bundles. (c) Possible kinetics of the interactions. CLIP-170 targets the dynein/dynactin complex to the MT, using phopho-LIS1 as an adapter (step 1); the motor complex, being in the proximity of the MT, associates with it (step 2); the initial static link to the MT provided by CLIP-170 is released by its hyperphosphorylation (step 3); and the retrograde dynein/dynactin-dependent movement of the cargo (endocytic vesicle, kinetochore, etc.) takes place (step 4). Tip1p, the CLIP-170 ortholog in S. pombe, has been reported by Brunner and Nurse (8) to act as an anticatastrophe factor. Based on the model depicted here, it also could be that CLIP-170 plays a similar role in eukaryotic cells by targeting MT-stabilizing factors as cargo to the plus tips. The model is based on that of Rickard and Kreis (54; reviewed in references 1a, 54a, and 75).