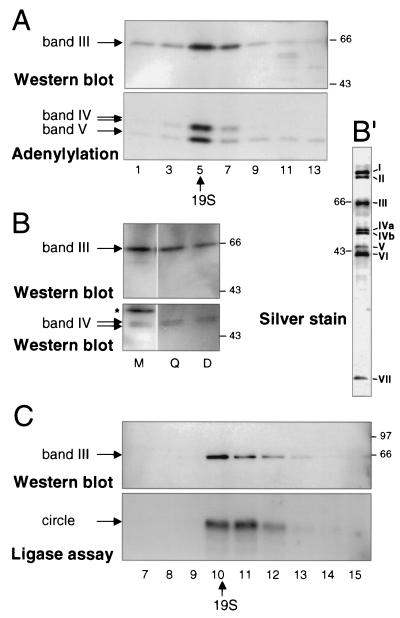
FIG. 2.
Band III copurifies with band IV. (A) Glycerol gradient sedimentation analysis of band III and bands IV and V. Fractions from mitochondrial extract (strain TREU 667; the two band IV isoforms are described in Materials and Methods) were resolved and analyzed by Western blotting for band III and by adenylylation for bands IV and V. Fraction 1 was at the bottom of the gradient, and fraction 14 was at the top; 19S was identified in a parallel glycerol gradient of thyroglobulin. There are inhibitors of band IV adenylylation high in the gradient (25). The same SDS gel, transferred to a membrane, was first exposed for adenylylation analysis and then used for Western analysis. The adenylylated proteins are not seen in the Western blot, because film was exposed for ∼10 s to score the chemiluminescence generated in the Western analysis, but 18 h was required to score the adenylylation. (B) SDS-PAGE gel analysis of band III and band IV in crude extract and partly and highly purified editing complex. The gel was loaded with 0.1 μl of crude mitochondrial extract (∼0.25 μg), 0.5 μl of the peak fraction after this extract had been put over a Q-Sepharose column (Q), and 5 μl of the peak fraction after this partially purified extract had been further purified over a DNA cellulose column (D) to yield the seven-polypeptide editing complex. The blot was sequentially probed with polyclonal antibodies against band IV (lower panel) and band III (upper panel). In crude mitochondrial extract, some cross-reacting proteins are observed (∗). Silver staining of proteins from a Q-Sepharose- and DNA cellulose-purified editing complex is shown in panel B′. (C) Such a Q-Sepharose- and DNA cellulose-purified seven-polypeptide complex had been subjected to glycerol gradient sedimentation by Rusché et al. (33), where the editing activities were shown to sediment at ∼20S; the assay of RNA ligase activity from these fractions is reproduced in the lower panel. The same glycerol gradient fractions were here reassayed for band III protein (upper panel). Sedimentation time for panel C was considerably shorter than that for panel A.