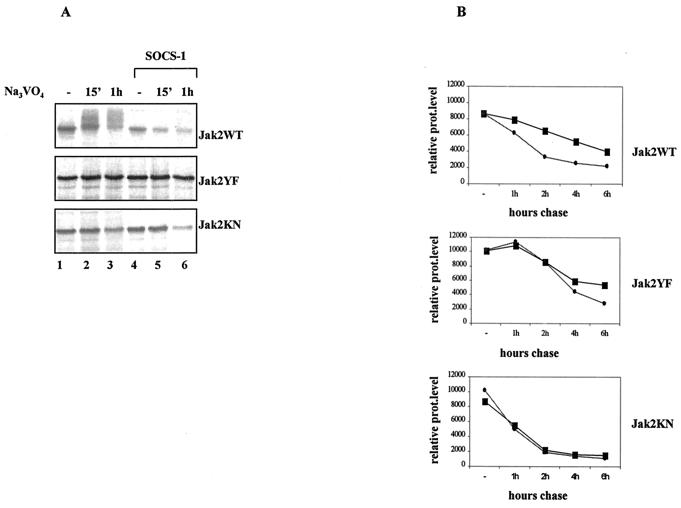
FIG. 7.
Phosphorylation-dependent proteasomal degradation of Jak2WT is enhanced by SOCS-1 coexpression. (A) SOCS-1 coexpression enhances the degradation of Jak2WT but not Jak2YF or Jak2KN in vivo. Cos-7 cells were transfected with Jak2 plasmid (3 μg) either alone or with the SOCS-1 plasmid (5 μg) and pretreated with proteasome inhibitor MG132 (20 μM) for 1 h before 35S metabolic labeling. The inhibitor was maintained throughout the pulse-labeling experiment. Cells were then treated with pervanadate (25 μM) and lysed in NP-40 lysis buffer supplemented with 25 μM MG132. Jak2 protein levels were analyzed by immunoprecipitation using an anti-HA antibody followed by autoradiographic detection. (B) Half-lives of different Jak2 mutants in the absence (square) or presence (circle) of SOCS-1 coexpression. Cos-7 cells were transfected with different Jak2 plasmids alone or together with the SOCS-1 plasmid and pulse-labeled with 35S for 15 min and chased for the indicated periods. Jak2 protein (prot) levels were analyzed by anti-HA immunoprecipitation followed by autoradiographic exposure. Images were scanned and quantified by using the Quan-Image5 program, and the relative protein amounts were adjusted so that time zero represents 100.