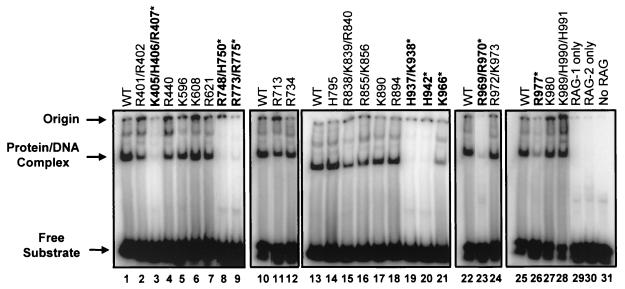
FIG. 2.
Mutations outside of the nonamer-binding domain in RAG-1 affect DNA binding. DNA binding was evaluated by electrophoretic mobility shift assays in which GST-tagged RAG-1 mutants coexpressed with GST-tagged RAG-2 were incubated with a radiolabeled 12-RSS oligonucleotide substrate. The positions of the free substrate, the protein-DNA complex, and the origin are indicated. Mutants with severe DNA-binding defects are in boldface type and are indicated by an asterisk. All lanes within each panel are from the same gel. Quantification was done by PhosphorImager analysis of the results of binding experiments with two independent protein preparations. WT, wild type. (Mutant K966 was previously shown to be proficient for RSS binding [30].)