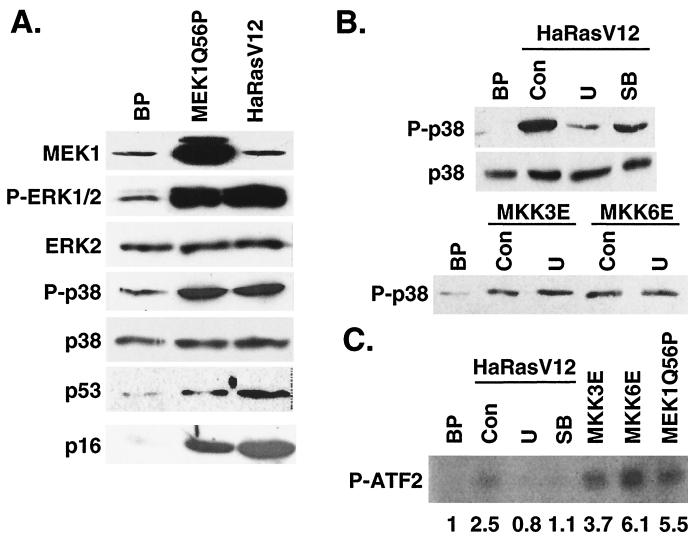
FIG. 8.
p38 activation by ras is mediated by an active MEK-ERK pathway. (A) Constitutively active MEK1 increases p38 phosphorylation. Shown are the results of Western blot analysis of BJ cells transduced with a vector control (BP), Ha-RasV12 (HaRasV12), or MEK1Q56P (MEK1Q56P) at day 6 postselection, indicating the protein levels of MEK1 (MEK1), phospho-ERK (P-ERK1/2), ERK2 (ERK2), phospho-p38 (P-p38), p38 (p38), p53 (p53), and p16INK4A (p16). (B) Inhibition of MEK-ERK activity prevented ras-induced p38 phosphorylation. Shown are the results of Western blot analysis of BJ cells transduced with a vector control (BP), Ha-RasV12 (HaRasV12), MKK3E (MKK3E), or MKK6E (MKK6E) in the presence of vehicle control (Con), U0126 (U), or SB203580 (SB) at day 10 postinfection, with the levels of phospho-p38 and p38 indicated. (C) Protein kinase activity of p38 immunoprecipitated from BJ cells transduced at PD 30 with a vector control (BP), Ha-RasV12 (HaRasV12) in the presence of vehicle control (Con), U0126 (U), or SB203580 (SB), constitutively active MKK3 (MKK3E) or MKK6 (MKK6E), or active MEK1 (MEK1Q56P) at day 10 postinfection. [γ-32P]ATP and GST-ATF2 were used as substrates. The numbers represent the relative amounts of phosphorylated ATF2 substrate after normalization to background. The signals were visualized and quantitated with a phosphorimager.