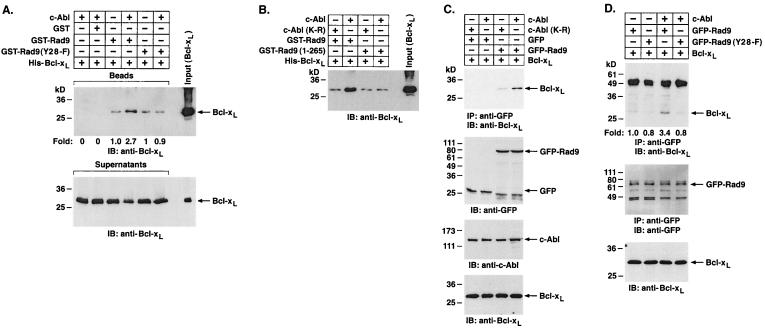
FIG. 5.
Tyrosine phosphorylation of Rad9 by c-Abl induces binding of Rad9 to Bcl-xL in vitro. (A) Glutathione beads containing GST, GST-Rad9, or GST-Rad9(Y28-F) were incubated with or without recombinant c-Abl and ATP for 30 min. The reaction mixtures were incubated with purified His-Bcl-xL protein for an additional 1 h. The beads (top) and supernatants (bottom) were analyzed by SDS-PAGE and immunoblotting (IB) with anti-Bcl-xL. Levels of Bcl-xL binding to GST-Rad9 were quantitated by densitometric scanning of signals. (B) Recombinant c-Abl and c-Abl(K-R) were incubated with GST-Rad9 or GST-Rad9 (1-265) for 30 min followed by incubation with the purified His-Bcl-xL protein for 1 h. Reaction products were separated by SDS-PAGE and analyzed by immunoblotting with anti-Bcl-xL. (C) 293T cells were cotransfected with 1 μg of pcDNA3-Bcl-xL and 3 μg of c-Abl, c-Abl(K-R), GFP vector, or GFP-Rad9. Anti-GFP immunoprecipitates (IP) were subjected to immunoblotting with anti-Bcl-xL (top). Cell lysates were also subjected to immunoblotting with anti-GFP (second from top), anti-c-Abl (third from top), or anti-Bcl-xL (bottom). (D) 293T cells were cotransfected with 1 μg of pcDNA3-Bcl-xL and 3 μg of c-Abl, GFP-Rad9, or GFP-Rad9(Y28-F). Anti-GFP immunoprecipitates were subjected to immunoblotting with anti-Bcl-xL (top) or anti-GFP (middle). Cell lysates were also subjected to immunoblotting with anti-Bcl-xL (lower). Levels of Bcl-xL binding to GFP-Rad9 were quantitated by densitometric scanning of the signals.