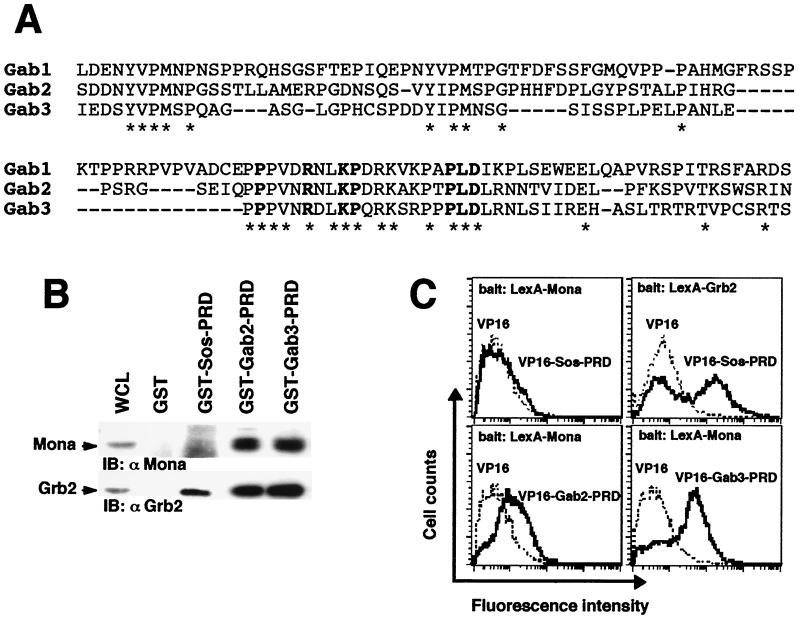
FIG. 1.
Gab2 and Gab3 are potential Mona partners. (A) Alignment of the carboxy-terminal PRDs of Gab family members. Bold characters show amino acid residues featuring the atypical SH3-binding domain as defined by Lock et al . (32). (B) The PRD is sufficient to mediate binding of Grb2 and Mona to either Gab2 or Gab3. Lysates from FD/Fms/Mona cells were incubated with purified, immobilized GST fused to protein segments encompassing Gab2- or Gab3-PRD. Bound proteins were run on an SDS-12% PAGE gel and transferred onto nitrocellulose membranes that were immunoblotted (IB) with anti-Mona (1:1,000) or anti-Grb2 (1:5,000) antibody (left panel). The GST-Sos-PRD fusion protein was used as a specificity control, known to bind Grb2 only (right panel). (C) Interaction between Mona and Gab proteins assessed in the yeast two-hybrid system. Bait and target proteins were expressed in yeast as described in Materials and Methods. Positive interactions of bait and target proteins were detected by fluorescence due to transcriptional activation of the GFP reporter gene. Data are representative of three independent experiments (except for Grb2, for which n = 2). For LexA-Mona experiments, average fluorescence intensities were 7.6 ± 1.0 (empty VP16), 8.0 ± 0.6 (VP16-Sos-PRD), 14.7 ± 1.3 (VP16-Gab2-PRD), and 44.2 ± 3.0 (VP16-Gab3-PRD). For LexA-Grb2 experiments, average fluorescence intensities were 9.0 (empty VP16) and 125.2 (VP16-Sos-PRD). Values are given as means ± standard errors of the means.