FIG. 3.
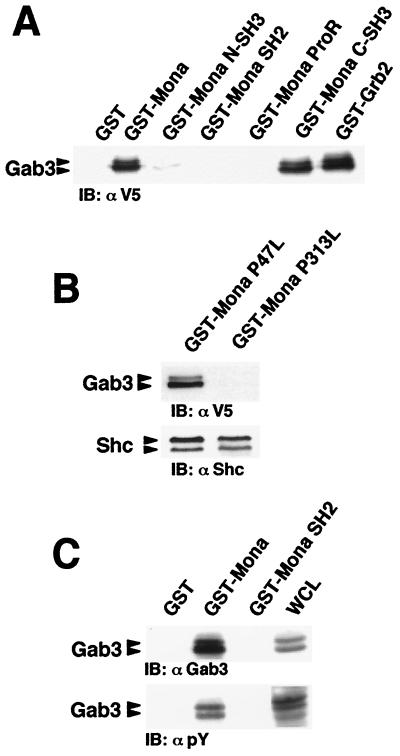
The carboxy-terminal Mona SH3 domain mediates binding to Gab3. (A) Lysates from FD/Fms/Gab3V5 cells were incubated with purified, immobilized GST fused to the full-length Mona or Grb2 protein or to the individual Mona domains, including amino-terminal SH3 domain (N-SH3), SH2 domain, ProR, and carboxy-terminal SH3 domain (C-SH3). Bound proteins were run on an SDS-12% PAGE gel and immunoblotted (IB) with anti-V5 (1:5,000) antibody. (B) Mona C-SH3 domain mutant fails to bind Gab3. Purified, immobilized GST fusion proteins containing full-length Mona protein harboring the P47L mutation in the amino-terminal SH3 domain or the P313L mutation in the carboxy-terminal SH3 domain were mixed with FD/Fms/Gab3V5 cell lysates. Bound proteins were analyzed on an SDS-12% PAGE gel by immunoblotting with anti-V5 or anti-Shc (1:2,000) antibody. (C) Mona SH2 domain does not bind to tyrosine phosphorylated Gab3. Cell lysates from M-CSF-stimulated FD/Fms/Gab3V5 cells (1 min 30 s, 37°C) were incubated with purified, immobilized GST fused with full-length Mona or Mona SH2 domain. WCL (50 μg) and protein complexes were separated by SDS-7.5% PAGE and immunoblotted with anti-Gab3 (1:100) or antiphosphotyrosine (αpY; 1:10,000) antibody.