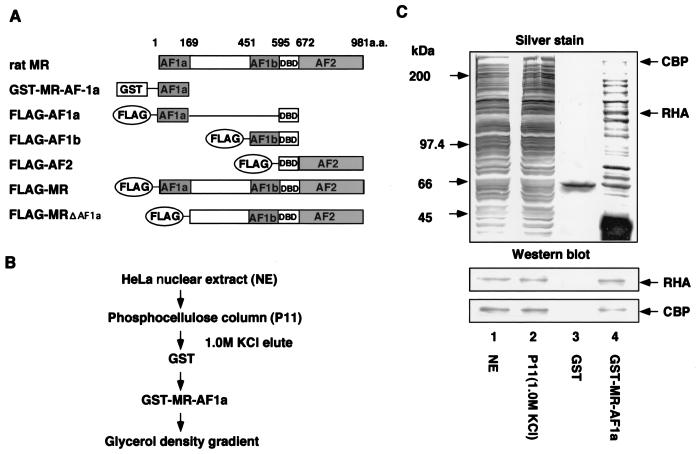
FIG. 1.
Purification and identification of MR AF-1a-binding complexes. (A) Schematic representation of rat MR activation domains and deletion mutants. AF-1a and AF-1b are ligand-independent activation regions within the rat MR A/B domain. GST-fused MR AF-1a (GST-MR-AF-1a) was used as bait for binding-complex purification. A DNA binding domain (DBD) was present in all rat MR deletion mutants (FLAG-AF1a, FLAG-AF1b, FLAG-AF2, and FLAG-MRΔAF1a). (B) Purification scheme for MR AF-1a interactants. Nuclear extracts from HeLa S3 cells were applied to P11 phosphocellulose columns. After extensive washes with wash buffer (20 mM Tris-HCl [pH 7.9], 0.2 mM EDTA, 0.05% NP-40, 10% glycerol, 0.5 mM PMSF, 1 mM DTT) containing 0.15 M KCl, bound proteins were eluted with wash buffer containing 1.0 M KCl. Eluted fractions were then incubated with immobilized GST-MR-AF-1a, and MR AF1a-interacting proteins were eluted by using N-lauryl sarcosine. (C) Identification of MR AF-1a interactants. The indicated fractions were subjected to SDS-PAGE followed by silver staining. Total HeLa S3 nuclear extracts (NE) (lane 1), a fraction eluted from a P11 column (P11) (lane 2), and eluted fractions from GST and GST-MR-AF-1a (lanes 3 and 4) are shown. Proteins eluted from GST-MR-AF-1a (lane 4) were identified by MS analysis. Lower panels show Western blotting for each fraction using antibodies against RHA or CBP.