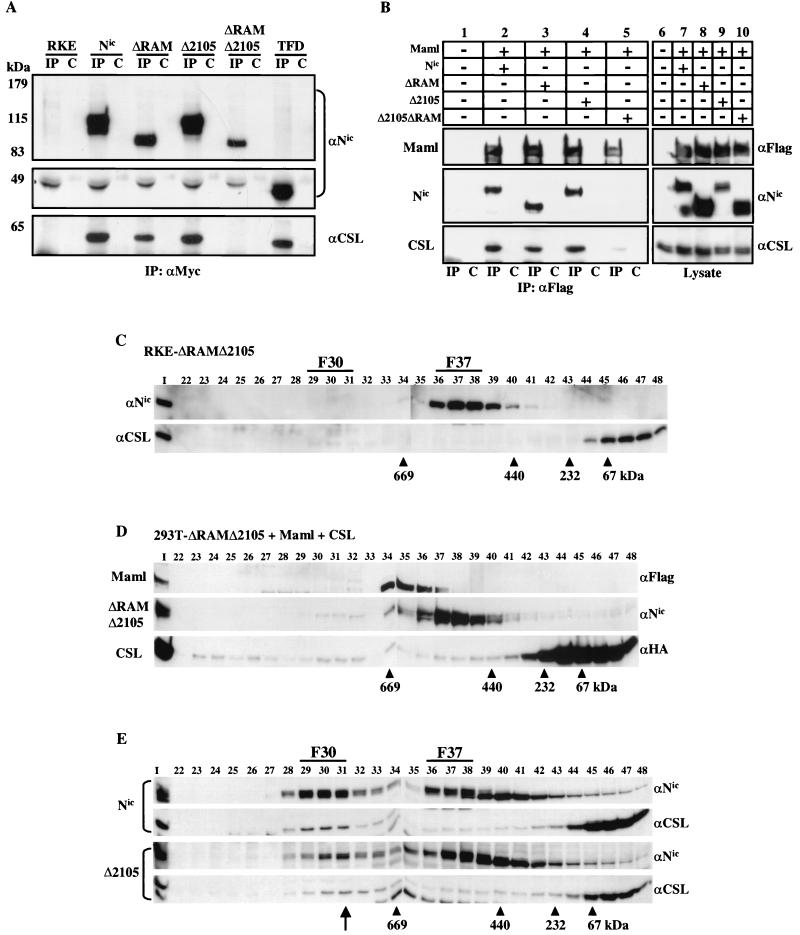
FIG. 6.
Deletion of the RAM domain and residues (amino acids 2105 to 2114) within the TFD results in loss of F30 formation. (A) Whole-cell lysates were made from the indicated RKE cell lines and were immunoprecipitated with either the anti-Myc antibody (lanes IP) or a nonspecific mouse IgG antibody control (lanes C). Proteins were detected with the indicated antibody. CSL is immunoprecipitated when the RAM domain or the Δ2105 region is present but not when both regions are deleted. Since the TFD is approximately 45 kDa, the membrane was split to be probed with both anti-Notch and anti-CSL antibodies. (B) ΔRAMΔ2105 is unable to appreciably associate with Maml and endogenous CSL in 293T cells. 293T cells were cotransfected with the indicated plasmids, and whole-cell lysates were immunoprecipitated with anti-Flag directed against Maml (lanes IP) or with a control mouse IgG (lanes C). Immunoprecipitated proteins were detected with the indicated antibodies (lanes 1 to 5).Expression of each protein was verified by Western blot analysis with the indicated antibodies (lanes 6 to 10). (C) ΔRAMΔ2105 derivative does not integrate into F30. Nuclear lysate from RKE cells expressing ΔRAMΔ2105 was fractionated by size exclusion chromatography. ΔRAMΔ2105 protein was visualized by Western blot analysis with bTAN15A (αNic). CSL protein was detected with anti-CSL polyclonal antisera. (D) ΔRAMΔ2105 is impaired for F30 reconstitution in 293T cells. 293T cells were cotransfected with the indicated expression vectors and processed as previously described. (E) A 10-residue deletion within the TFD that abolishes transformation also disrupts protein complex formation. Gel filtration profiles for Nic and Δ2105 are compared. Proteins were detected by Western blotting with the indicated antibodies (right). Note that the position of F30 in the Δ2105 cell line is shifted down one column fraction compared to the Nic profile (arrow).