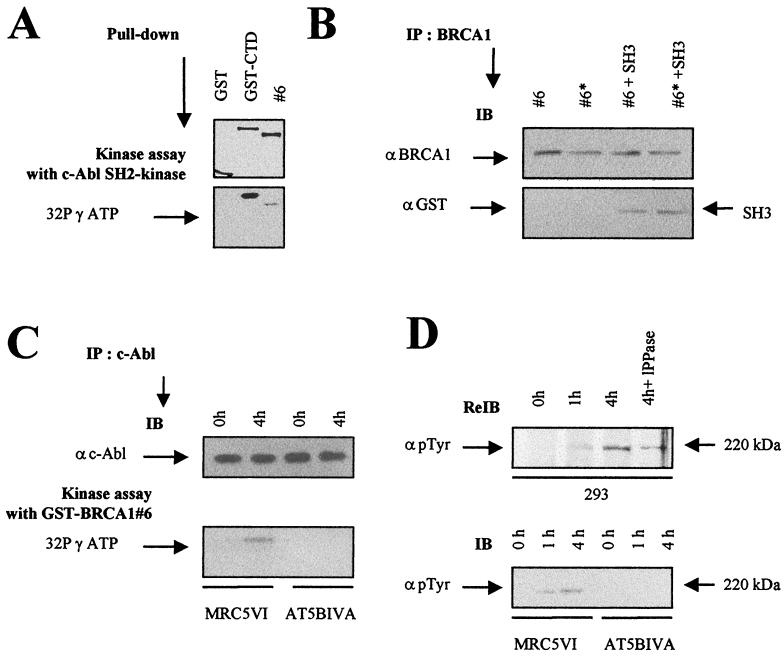
FIG. 6.
C-terminal BRCA1 is a substrate for c-Abl tyrosine kinase. (A) c-Abl tyrosine kinase assay of GST-BRCA1#6 using c-Abl SH2 kinase. Purified c-Abl SH2 kinase (1 μg) was incubated with equal amounts (5 μg) of GST, GST-CTD, and GST-BRCA1#6 fusion proteins as indicated in Materials and Methods (top). The resulting GST adsorbates were subjected to SDS-PAGE and phosphorimager analysis (bottom). (B) SH3 binding of c-Abl-phosphorylated GST-BRCA1#6 substrates. Equal amounts of GST-BRCA1#6, whether tyrosine phosphorylated (#6*) or not (#6) as described below, were subjected to BRCA1 immunoprecipitation (IP) using anti-BRCA1 (αBRCA1; Ab-5 and Ab-3) antibodies. The substrates were mixed with equal amounts of eluted GST-SH3 proteins. The presence of the GST-SH3 proteins in the adsorbates was examined by immunoblotting (IB) using anti-GST (αGST) antibody. (C) c-Abl tyrosine kinase assay of GST-BRCA1#6 using endogenous c-Abl. c-Abl immunoprecipitates of nuclear extracts from MRC5VI and AT5BIVA (ATM−/−) cells exposed to 20 Gy were collected after irradiation at the indicated times (top) in a kinase assay with the GST-BRCA1#6 fusion protein (5 μg) as a substrate. The resulting GST adsorbates were subjected to SDS-PAGE and phosphorimager analysis (bottom). (D) The nitrocellulose membrane used for BRCA1 immunoblotting of 293 cells extracts shown in Fig. 3A was rehybridized by anti-p-Tyr (α p Tyr) tyrosine antibody (top). Nuclear extracts from unirradiated and irradiated MRC5VI and AT5BIVA cells were subjected to p-Tyr immunoblotting (bottom).