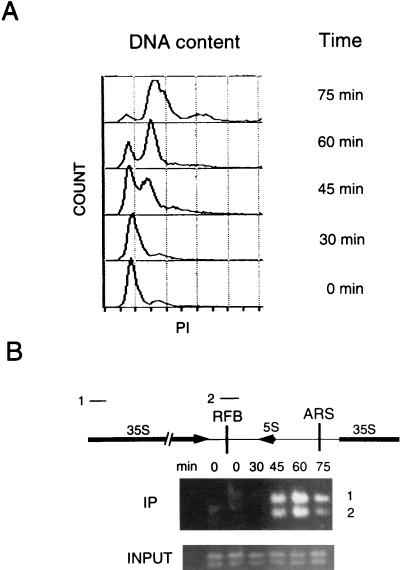
FIG. 4.
ChIP assays with synchronized cells demonstrate that Dna2p associates with rDNA maximally during S phase. (A) Cells were synchronized with mating pheromone and released as described in Materials and Methods. Flow cytometry was performed as described previously (12). Propidium iodide (PI) measures DNA content. (B) ChIP assays were performed with a strain in which the DNA2 open reading frame was precisely replaced by DNA2 fused to 9 myc tags in strain W303 pep4bar1, such that the fusion protein is expressed under the control of its endogenous promoter (12). The strain shows the same growth rates as the parental strain and is designated DNA2TMTH. Cross-linking, cell lysis, shearing, immunoprecipitation, and PCR amplification were carried out as described in Materials and Methods. The DNAs amplified from the anti-myc 9E10 monoclonal antibody immunoprecipitates are labeled IP and correspond to DNAs associated with Dna2p. The PCR products 1 and 2 correspond to the rDNA regions labeled 1 and 2 in the diagram. Control PCRs using as template the total genomic DNA in the extracts showed that each fragment amplified equally at each time point (labeled INPUT). The single-copy ARS1 probe showed no signal, and there is no internal control for rDNA, which has a very high copy number. The data shown are from a 25-cycle amplification (see Materials and Methods). The same procedure, carried out on a strain in which DNA2 was not fused to myc, yielded no PCR product (data not shown).