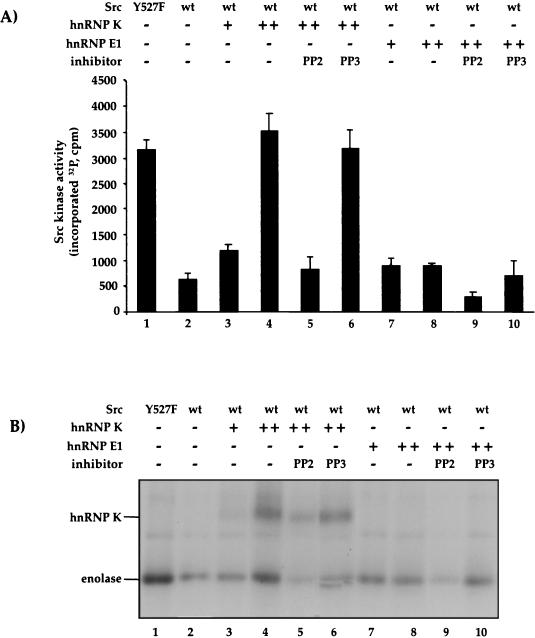
FIG. 2.
hnRNP K but not hnRNP E1 activates Src catalytic activity in vitro. Wild-type Src (wt) and the constitutively active Src(Y527F) mutant as a positive control were immunopurified from transfected human 293 HEK cells, and equal amounts were assayed with an excess of the substrate enolase in the presence of [γ-32P]ATP, incubated with increasing amounts (125 [+] and 375 [++] ng) of purified hnRNP K (lanes 3 and 4) or hnRNP E1 (lanes 7 and 8). The Src inhibitor PP2 (lanes 5 and 9) and the control PP3 (lanes 6 and 10) were added at a concentration of 1 μM. Phosphorylation of enolase and hnRNP K was analyzed by autoradiography (an example is shown in panel B). (A) The bars show the sum of the hnRNP K and enolase phosphorylation signals with the standard deviation observed in three repeat experiments.