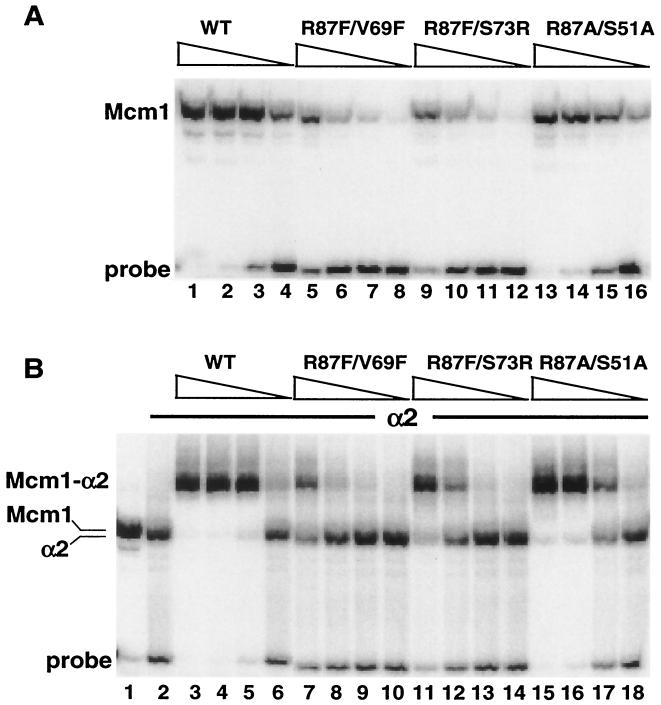
FIG. 7.
DNA binding of radical double-mutation mcm1-encoded proteins in combination with α2. (A) Binding affinity of wild-type (WT) Mcm1 (lanes 1 to 4) and mutant proteins with the R87F/V69F (lanes 5 to 8), R87F/S73R (lanes 9 to 12), and R87A/S51A (lanes 13 to 16) mutations to the STE6 α2-Mcm1 site. The proteins were diluted by fivefold dilutions from a concentration of 4 × 10−10 M (lanes 1, 5, 9, and 13). (B) Binding affinity of wild-type Mcm1 (lanes 3 to 6) and mutant proteins with the R87F/V69F (lanes 7 to 10), R87F/S73R (lanes 11 to 14), and R87A/S51A (lanes 15 to 18) mutations in the presence of 4.7 × 10−7 M α2. The proteins were diluted by fivefold dilutions from a concentration of 4 × 10−10 M (lanes 3, 7, 11, and 15). In panel B, lane 1 shows the position of wild-type Mcm1 binding in the absence of α2 and lane 2 shows the position of α2 binding in the absence of Mcm1.