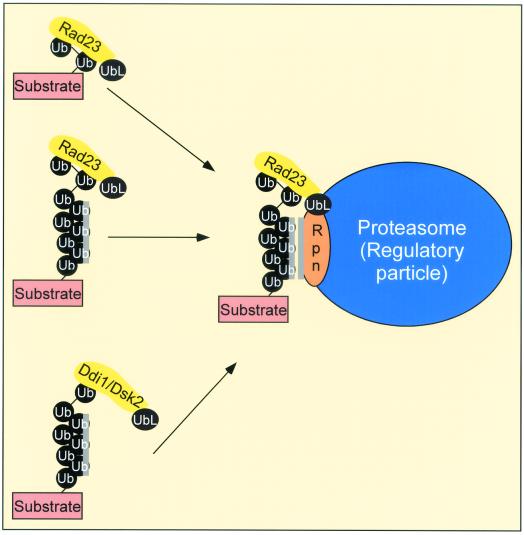
FIG. 7.
Model. Rad23 translocates ubiquitinated proteins to the proteasome. We provide evidence that Rad23 can interact with substrates that are ligated to multi-Ub chains (black circles). The interaction with ubiquitinated substrates could transiently inhibit further multi-Ub chain assembly and thereby stabilize the protein. However, subsequent delivery of the ubiquitinated substrate to specific proteasome-associated multi-Ub chain binding factors, such as Rpn10 (Rpn), could initiate degradation. Rad23 and Rpn10 may recognize different determinants in a multi-Ub chain, and consequently both proteins could interact simultaneously with substrate-linked multi-Ub chains. The interaction between Rpn10 and the multi-Ub chain is mediated by hydrophobic interactions, indicated by the stripe next to the multi-Ub chain and Rpn10. In contrast, Rad23 might interact with the distal Ub moieties in a multi-Ub chain. We speculate that proteasome-associated E2 and E3 factors could further ubiquitinate the substrate to promote efficient degradation. This scheme also anticipates that other UBA- and UbL-containing proteins, including Ddi1 and Dsk2, perform similar roles in the delivery of proteolytic substrates and regulators to the proteasome.