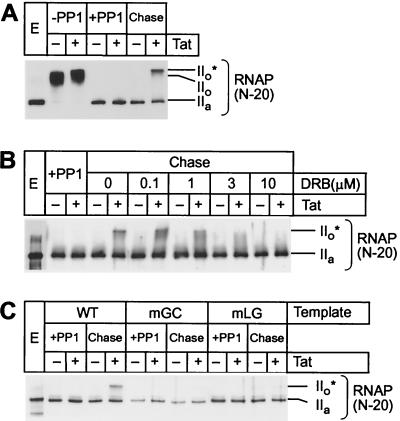
FIG. 2.
Tat and TAR stimulate hyperphosphorylation of CTD in transcription elongation complexes. (A) Rephosphorylation by CDK9. Elongation complexes assembled on the pW1 templates were dephosphorylated by PP1 treatment (Pol IIa). Chase of the dephosphorylated complexes from the LacR site in the presence of all four nucleotides and IPTG permits rephosphorylation of the RNA polymerase CTD (Pol IIo*) in the presence (+) of 20 ng of Tat but not in the absence (−) of Tat. (B) Inhibition of CDK9 by DRB. Between 0 and 10 μM DRB was included in the chase reactions containing dephosphorylated elongation complexes. Transcription was performed on the pW1 DNA templates in the absence (−) and presence (+) of 20 ng of Tat. (C) Activation of CDK9 by Tat and TAR. Elongation complexes were assembled on templates carrying wild-type TAR (WT) or mutant TAR elements in the Tat-binding site (mGC) or in the CycT1-binding site (mLG) in the absence (−) or presence (+) of 20 ng of Tat. After dephosphorylation of RNA polymerase CTD, the complexes were chased as described above.