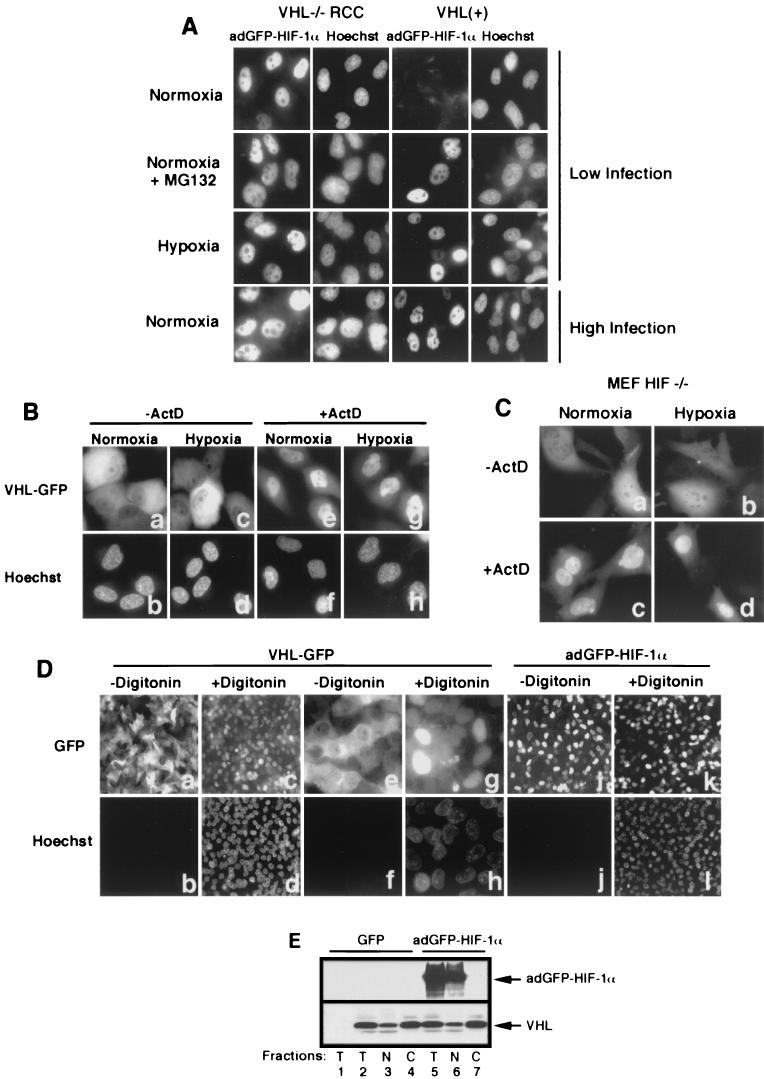
FIG.2.
Subcellular localization of VHL and adGFP-HIF-1α is independent from each other. (A) adGFP-HIF-1α localize to the nucleus. Fluorescence analysis of VHL−/− RCC cells and the VHL(+) WT-7 stable cell line expressing adGFP-HIF-1α in normoxia at low levels of infection (first row) and of normoxic cells in the presence of proteasome inhibitor MG132 (second row) or in hypoxia (third row). Normoxic VHL−/− RCC and VHL(+) cells infected with high titers of adGFP-HIF-1α are shown in the last row. (B) Transcription-dependent shuttling of VHL is unaffected by hypoxic treatment. Fluorescence analysis of VHL−/− RCC cells expressing VHL-GFP in normoxia or hypoxia (4 h) in the absence or the presence of ActD (10 μg/ml) for 2 h. (C) Transcription-dependent shuttling of VHL occurs in the absence of substrate HIF-α. Fluorescence analysis of HIF−/− mouse embryonic fibroblasts expressing VHL-GFP in normoxia or hypoxia (4 h) in the absence or the presence of ActD (10 μg/ml) for 2 h. (D) Digitonin-permeabilization system to achieve nuclear-cytoplasmic fractionation of cells. VHL-GFP-expressing VHL−/− RCC cells were either not treated (a and b and, at a higher magnification, e and f) or treated for 5 min with 50 μg of digitonin/ml (c and d and, at a higher magnification, g and h) and observed for GFP fluorescence (a, c, e, and g) or for cell-impermeable Hoechst stain 33258. Notice the complete loss of cytoplasmic VHL-GFP signal but the retention of nuclear signal in digitonin-treated cells and that essentially all of the nuclei of permeabilized cells have been stained with Hoechst. Panels j to l show that nuclear adGFP-HIF-1α remains in the nuclear compartment after digitonin treatment. (E) VHL steady-state localization is independent of levels of adGFP-HIF-1α in normoxic cells. VHL(+) cells were either infected with GFP adenovirus or with high levels of adGFP-HIF-1α virus. Biochemical subcellular fractionation was performed with the digitonin system to obtain total (T), nuclear (N), and cytosolic (C) fractions, which were analyzed by Western blot with anti-M2 and anti-HIF-1α antibody. A negative control is present in the first lane and represents VHL−/− RCC infected with GFP virus.