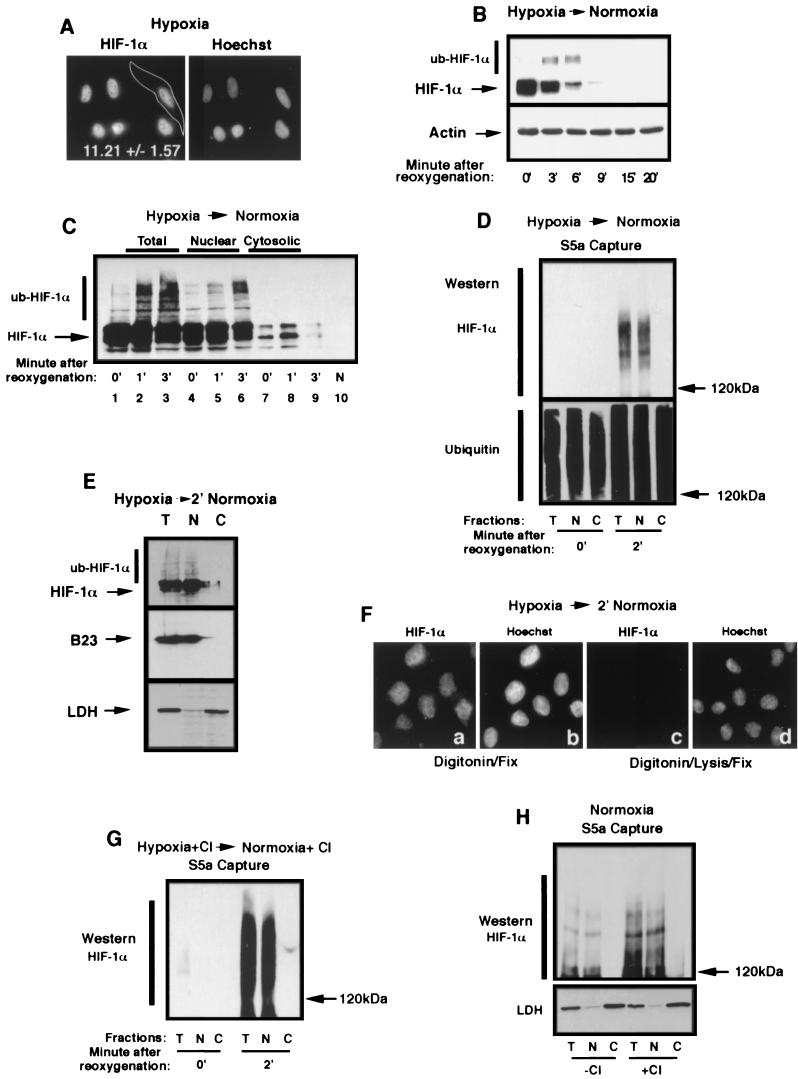
FIG.3.
Ubiquitination of HIF-1α occurs in the nuclear compartment. (A) HIF-1α localizes to the nuclear compartment of hypoxic HeLa cells. HeLa cells grown on coverslips were incubated for 8 h in hypoxia prior to fixation with 1% formaldehyde and staining with an anti-HIF-1α antibody and a secondary Texas red-labeled anti-mouse antibody. A CCD-captured image was analyzed as described in Materials and Methods for the nuclear/cytoplasmic ratio of the distribution of the fluorescence signal. The mean ± the standard deviation of 32 cells of three independent experiments is shown. (B) HIF-1α is efficiently degraded upon the return of hypoxic cells to normoxia. HeLa cells were incubated for 8 h in hypoxia prior to a return to a normoxic incubator for the indicated time. Cells were lysed in 4% SDS-PBS and analyzed by SDS-8% PAGE, followed by Western blotting with an anti-HIF-1α antibody. Notice the appearance of higher-molecular-weight bands at 3 and 6 min, which is most likely ub-HIF-1α. (C) ub-HIF-1α appears promptly in the nucleus after reoxygenation of hypoxic cells. HeLa cells were incubated in hypoxia for 8 h (0′) prior to being transferred to an oxygenated environment at 37°C for 1 and 3 min. Samples from each time point were submitted to subcellular fractionation, and the resulting cell fractions (total, nuclear, and cytosolic) were analyzed by SDS-8% PAGE, followed by Western blotting with an anti-HIF-1α antibody. Normoxic (N) HeLa cell lysate is shown in lane 10. (D) ub-HIF-α is found strictly in the nuclear compartment upon reoxygenation. Capture of polyubiquitinated proteins with agarose-GST-S5a beads was carried out in lysates of total (T), nuclear (N), or cytosolic (C) fractions of hypoxic or reoxygenated (2 min) HeLa cells. The captured polyubiquitinated proteins were analyzed by SDS-6% PAGE, followed by Western blot with an anti-HIF-1α or anti-ubiquitin antibody. (E) Reoxygenated HeLa cells were treated with digitonin, and nuclear or cytosolic fractions were blotted with an anti-HIF-1α antibody, an anti-LDH antibody, or an anti-B23 antibody. (F) HIF-1α can be detected solely in the nucleus of digitonin-treated cells after a return of hypoxic cells to normoxia. Hypoxic HeLa cells grown on coverslips were transferred to an oxygenated incubator for 2 min prior to permeabilization with digitonin for 5 min at 4°C. Permeabilized cells were either fixed with 1% formaldehyde (a and b) or treated with lysis buffer prior to fixation (c and d). Fixed and permeabilized cells were staining with an anti-HIF-1α antibody and a secondary Texas red-labeled anti-mouse antibody (a and c) or with Hoechst stain (b and d). Note that the specific HIF-1α signal can be detected only in the nuclear compartment in panel a and is completely extractable with the lysis buffer (see panel c). (G) ub-HIF-1α can only be detected in the nuclear compartment in proteasome-treated and reoxygenated HeLa cells. HeLa cells were treated for 1 h with proteasome inhibitors prior to reoxygenation and cells were treated as described for panel D to detect ub-HIF-1α. (H) ub-HIF-1α are found solely in the nucleus of normoxic cells. Normoxic HeLa cells treated (+) or not (−) with calpain inhibitor (CI) were fractionated into total (T), nuclear (N), and cytosolic (C) lysates, which were then subjected to agarose-GST-S5a immunoprecipitation and anti-HIF-1α Western blot analysis. The bottom panel shows the fractions blotted with anti-LDH.