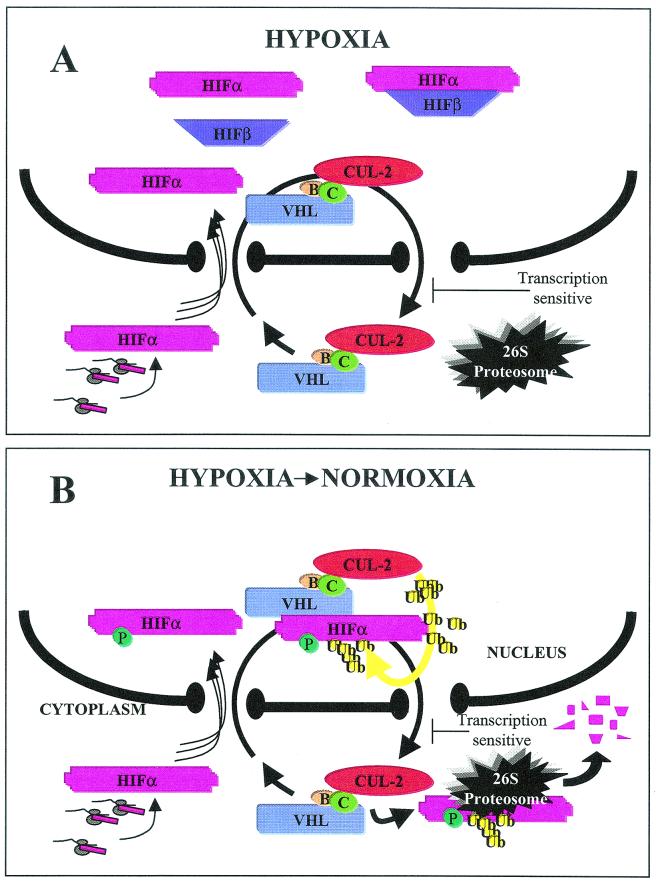
FIG. 8.
Model linking VBC/Cul-2 nuclear-cytoplasmic trafficking and oxygen-dependent ubiquitination and degradation of HIF-α. (A) In hypoxic conditions, HIF-α (pink) is stabilized and is immediately imported into the nucleus, where it binds to its partner HIF-β (purple) to form the HIF transcription factor that transactivates hypoxia-inducible genes such as vascular endothelial growth factor and glucose transporter-1. VBC/Cul-2 engages in a constitutive nuclear-cytoplasmic shuttle unaffected by oxygen tension or levels of substrate HIF-α. (B) Upon a return to normoxia, HIF-α incurs a posttranslational modification in the nuclear compartment, which is most likely the hydroxylation of proline of the ODD domains since ubiquitination is preventable by treatment with DMOG. Nuclear HIF-α binds to VHL and undergoes Cullin-2-mediated ubiquitination (yellow) prior to being exported to the cytoplasm for 26S proteasomal degradation. Nuclear HIF-α embarks on the VBC/Cul-2 “merry-go-round” depending upon its ability to assemble with nuclear VHL. The proposed model is applicable for hypoxic cells that are returned to an oxygenated environment. As for cells maintained in normoxia, we cannot totally exclude cytoplasmic ubiquitination and degradation of HIF-α. However, we prefer a model by which HIF-α in normoxia is rapidly imported for nuclear ubiquitination and reexported to the cytoplasm in a manner similar to that observed for reoxygenated cells.