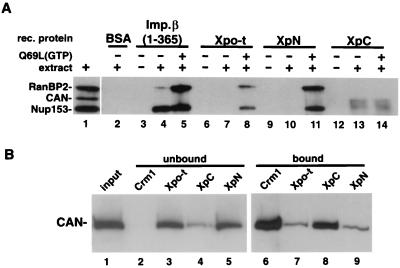
FIG. 7.
Nucleoporin binding to Xpo-t, XpN, and XpC. (A) The indicated recombinant (rec.) proteins were cross-linked to resin at high density (see Materials and Methods) and used for affinity binding. Affinity resin containing the different proteins was mixed with buffer (lanes 3, 6, 9, and 12), buffer containing Xenopus egg extract (lanes 2, 4, 7, 10, and 13), or extract supplemented with 5 μM RanQ69L loaded with GTP (lanes 5, 8, 11, and 14) and rotated at 4°C for 3 h. The resin was then collected and washed, and the bound proteins were eluted with SDS sample buffer and separated by SDS-6% PAGE and detected by Western blotting using MAb414. In the presence of RanGTP, Xpo-t and XpN can pull down both Nup153 and RanBP2/Nup358 (lanes 8 and 11). By contrast, XpC affinity resin can select CAN/Nup214, and this binding is RanGTP independent (lanes 13 and 14). (B) WGA-Sepharose affinity-purified proteins from Xenopus egg extracts (lane 1) were mixed with resin containing the indicated recombinant proteins and 25% of the unbound fractions (lanes 2 to 5) and bound fractions (lanes 6 to 9) were separated and detected as in panel A by using MAb414. For CRM1 both RanQ69L(GTP) and BSA-NES were included in the binding reaction to promote export complex formation.