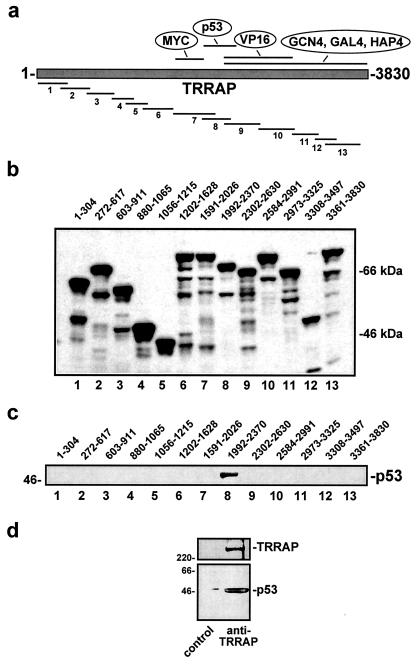
FIG. 7.
p53 directly binds to TRRAP in vitro and in vivo. (a) Fragments of TRRAP spanning the entire protein were expressed as GST fusions in Escherichia coli. The p53 binding domain defined in panel c is indicated. (b) Purified GST-TRRAP fusion proteins were resolved by SDS-PAGE, blotted, and probed for GST to document relative expression levels and confirm predicted molecular weights. The TRRAP amino acids encompassed in each fusion protein are indicated. (c) After purification, GST-TRRAP fusion proteins were used as bait in in vitro pull-down reactions. FLAG-tagged p53 expressed and affinity purified from baculovirus-infected cells served as the target protein in these reactions. After a washing, binding reaction mixtures were resolved by SDS-PAGE, blotted, and probed for p53. (d) Nondenaturing lysates of 293 cells were subjected to immunoprecipitation using antibodies directed against TRRAP or a control antibody. Precipitates were resolved, blotted, and probed for both TRRAP (top) and p53 (bottom). Migration of size markers is indicated at left in kilodaltons.