FIG. 4.
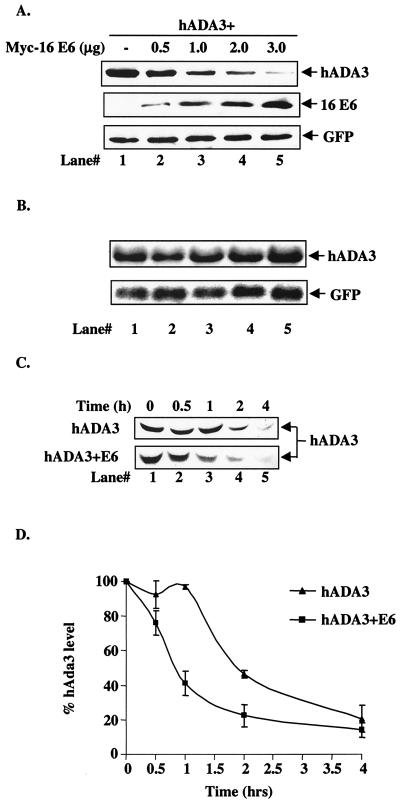
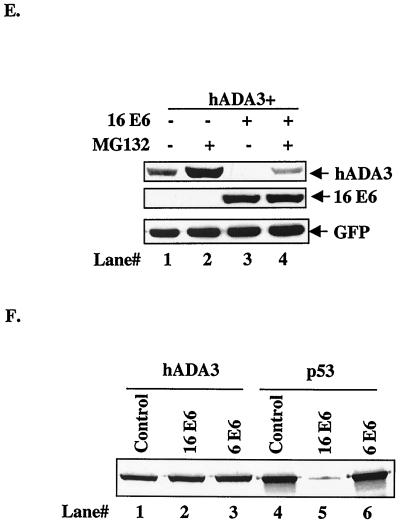
Reduction of hADA3 protein levels upon coexpression of HPV16 E6. (A) A total of 5 × 105 293T cells per 100-mm dish were transfected with 0.5 μg each of pCR3.1-FLAG-hADA3 either alone or together with increasing amounts of pEF-myc-HPV16 E6, as indicated. Five hundred nanograms of GFP DNA was included to assess the transfection efficiency. Total amount of DNA was kept constant by adding vector DNA. Cell lysates were prepared at 48 h posttransfection, and 30-μg aliquots of lysate protein were subjected to anti-FLAG immunoblotting (upper panel), followed by anti-myc (middle panel) and anti-GFP (lower panel) reblotting. (B) Duplicate plates of the transfections shown in panel A were used to isolate total cellular RNA, and 20-μg aliquots were used for Northern blotting with a 32P-labeled 1.3-kb hADA3 cDNA probe, followed by autoradiography (upper panel). A GFP cDNA probe was included in the hybridization mix as a control (lower panel). (C) Pulse-chase analysis of hADA3. 293T cells were transfected with 0.5 μg of pCR3.1-hADA3 alone or together with 2 μg of vector or pCR3.1-HPV16 E6. After 48 h, cells were metabolically pulse-labeled with [35S]methionine plus [35S]cysteine for 30 min and chased for the indicated time periods (shown in hours). Equal amounts of radiolabeled lysates (based on the counts per minute) were immunoprecipitated with anti-hADA3 antiserum and resolved by SDS-PAGE, followed by fluorography. A representative experiment is shown. (D) The amount of hADA3 was calculated by densitometry, and the percentage of hADA3 left was plotted against time indicated. The mean ± the standard deviation (SD) of three independent experiments is shown. (E) 293T cells were transfected with 0.5 μg of pCR3.1-FLAG-hADA3 alone or with 2.5 μg of pEF-myc-16 E6. At 20 h after transfection, the cells were either mock treated or treated with MG132 (50 μM) for 4 h. Immunoblotting was performed as for panel A. (F) Lack of in vitro degradation of hADA3 by E6. The 35S-labeled hADA3, p53, HPV6 E6, and HPV16 E6 proteins were generated by in vitro translation in rabbit reticulocyte lysates in the presence of [35S]cysteine (E6) or [35S]methionine (ADA3 and p53). Aliquots of 35S-labeled hADA3 (lanes 1 to 3) or p53 (lanes 4 to 6) were incubated with water-primed lysate (control; lanes 1 and 4) or HPV16 E6 (lanes 2 and 5) or HPV6 E6 (lanes 3 and 6) proteins for 15 h at 30°C. The hADA3 or p53 remaining at the end of the incubation period was resolved by SDS-PAGE and visualized by fluorography.