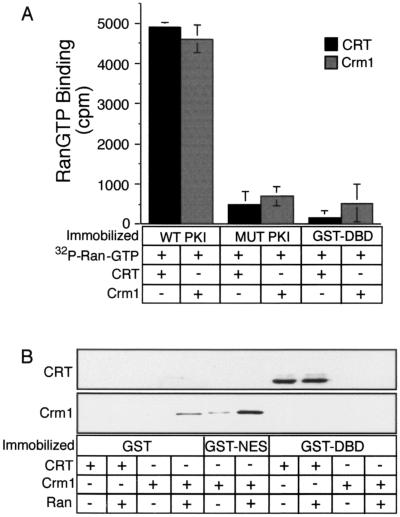
FIG. 1.
Formation of export complexes involving CRT and Crm1. (A) The incorporation of RanGTP into complexes containing CRT and Crm1 was assayed using Ran preloaded with [γ-32P] GTP. Target proteins were immobilized in microtiter wells (500 ng/well), and CRT or Crm1 (5 μg each) was added to each well together with 2 × 104 cpm of radiolabeled RanGTP. Following incubation for 1 h at room temperature, the wells were washed four times and the bound fractions were released and assayed by scintillation counting. (B) RanGTP is not a cofactor for CRT binding to the DBD. Target proteins (1 μg each) were immobilized on glutathione beads, and CRT or Crm1 (500 ng each) was added to each sample in the absence or presence of Ran (1 μg) preloaded with cold GTP. The samples were mixed end over end for 2 h at room temperature, washed three times, eluted, and analyzed by immunoblotting with antibodies to CRT and Crm1. These data show that, like Crm1, NES recognition by CRT involves RanGTP. In contrast, DBD recognition by CRT does not involve RanGTP.