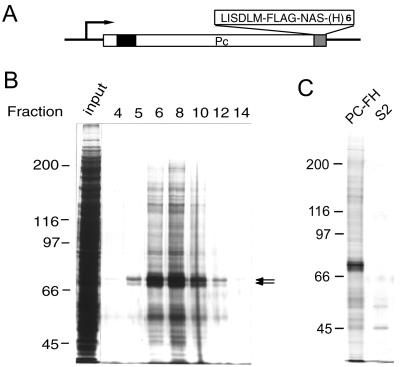
FIG. 1.
Purification of tagged Pc-G complex. (A) Structure of the epitope-tagged Pc construct. The chromodomain is indicated by a solid box. The C-terminal FLAG epitope and hexahistidine tags are labeled FLAG and (H)6, respectively. The extra amino acid residues introduced by the linker sequences are shown in single letter code. (B) Silver staining of eluted proteins. Aliquots (3.5 μl) of proteins from the column input and different fractions were resolved on an SDS-7% polyacrylamide gel. The fraction numbers are indicated at the top of each lane and are consistent with those in Fig. 2D and 3B. The position of PC is indicated by the arrows. Note that several discrete protein bands can be resolved in this region on high-resolution gels. (C) Specific association of Pc-G proteins. The eluted fractions prepared from PC-FH or S2 cells were resolved on an SDS-7% polyacrylamide gel. Only a small number of nonspecific proteins were eluted from S2 cell extracts.