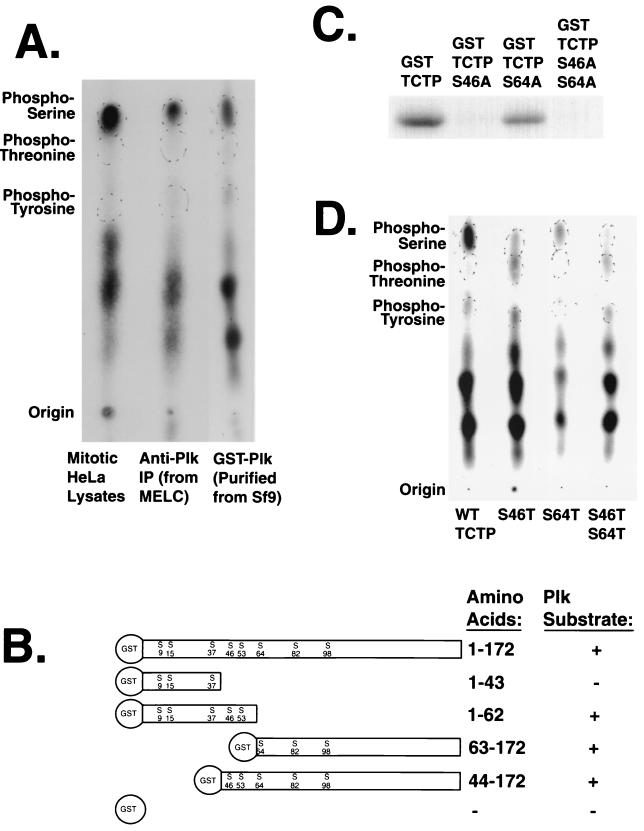
FIG. 4.
Plk phosphorylates TCTP on two serine residues. (A) Both Plk and mitotic lysates phosphorylate TCTP on serine residues. Partial acid hydrolysis followed by phosphoamino acid analysis was performed on GST-TCTP phosphorylated by GST-Plk purified from baculovirus-infected Sf9 cells, immunoprecipitated Plk from nocodazole-treated MEL cells, and crude lysate from nocodazole-arrested HeLa cells. (B) Deletion mapping of in vitro Plk phosphorylation sites in TCTP indicates more than one phosphorylation site. (C) Serine-to-alanine point mutant analysis indicates that Plk phosphorylates TCTP on serines 46 and 64 in a hierarchical fashion. GST-TCTP and mutant fusion proteins were phosphorylated by Sf9-produced GST-Plk in separate in vitro kinase reactions. (D) Serine-to-threonine mutations in TCTP support the two-Plk-phosphorylation-site hypothesis. TCTP mutants with combinations of serines and threonines at resides 46 and 64 were phosphorylated by GST-Plk and subjected to phosphoamino acid analysis. The lack of apparent Plk phosphorylation on the Ser64Thr residue indicates a specificity for serine at this site.