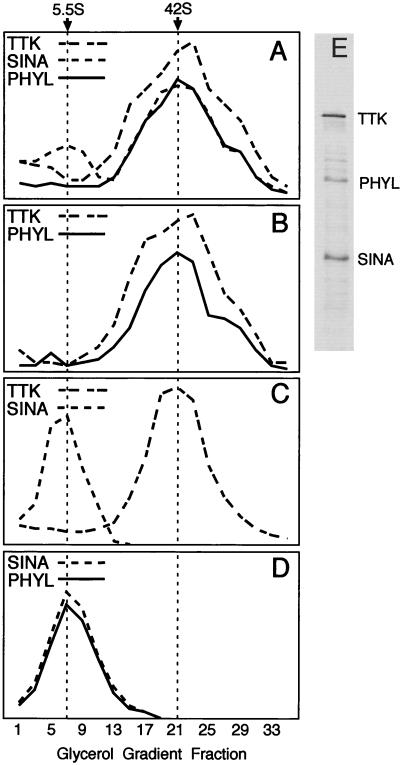
FIG. 3.
Glycerol gradient sedimentation analysis of Sina, Phyl, and Ttk. 35S-labeled proteins were incubated in binding reactions and subsequently sedimented as described in Materials and Methods. Thirty-five fractions were taken from each gradient and subjected to SDS-PAGE, followed by autoradiography. Protein levels in each fraction were quantitated by laser scanning densitometry and are graphed in arbitrary density units. In a parallel gradient, molecular weight standards were sedimented, and portions of each fraction were analyzed by SDS-PAGE. Svedberg coefficients were determined by comparison of the labeled protein peak in a gradient to the sedimentation profile of protein standards. Svedberg coefficients corresponding to gradient peaks are indicated at the top of the figure. (A) Binding reaction containing Sina, Phyl, and Ttk. (B) Binding reaction containing Phyl and Ttk. (C) Binding reaction containing Sina and Ttk. (D) Binding reaction containing Sina and Phyl. (E) SDS-PAGE analysis of labeled Sina, Phyl, and Ttk proteins loaded onto gradients.