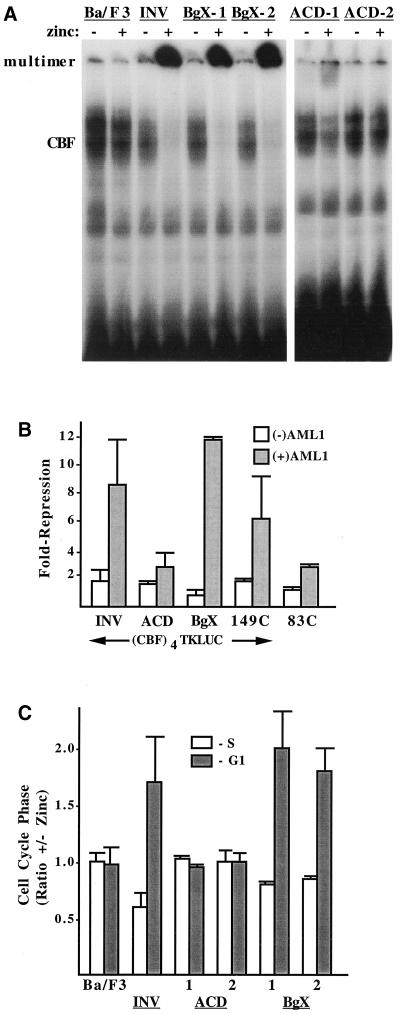
FIG. 4.
Deletion of the ACD prevents INV from being detected as a nuclear monomer or multimer in a gel shift assay, from inhibiting CBF DNA binding and transactivation, and from slowing G1 to S cell cycle progression. (A) Nuclear extracts prepared from parental Ba/F3 cells and from Ba/F3-INV, -BgX-1, -BgX-2, -ACD-1, and -ACD-2cells, cultured with and without zinc for 48 h, were subjected to the gel shift assay with a radiolabeled probe from the myeloperoxidase gene containing a consensus CBF DNA-binding site. A total of 12 μg of nuclear extract was employed for each binding assay. The locations of gel shift species containing endogenous CBF or multimeric INV or INV variants are shown. (B) 293T cells were transiently transfected with 750 ng of p(CBF)4TKLUC, 50 ng of pCMV (open bars), or pCMV-AML1B (shaded bars), and 50 ng of pCMV, pCMV-INV, pCMV-INV(ΔACD), pCMV-INV(ΔBgX), pCMV-INV(149C), or pCMV-INV(83C). Luciferase activities were determined 2 days later. pCMV-AML1B activated the reporter 3.1- to 6.6-fold (not shown). Repression by the indicated proteins represents activity relative to the reporter in the presence of either 100 ng of pCMV (open bars) or 50 ng of pCMV and 50 ng of pCMV-AML1B (shaded bars). Results represent mean and standard error from four determinations. (C) The indicated cell lines were subjected to cell cycle analysis after culture with and without zinc for 48 h. The proportion of cells in S or in G1 in the presence of zinc divided by the proportion in each cell cycle phase in the absence of zinc was determined by bromodeoxyuridine-propidium iodide analysis and is shown for each cell line (mean and standard error of two determinations). A higher ratio indicates a greater proportion in the presence of zinc.