Figure 2.
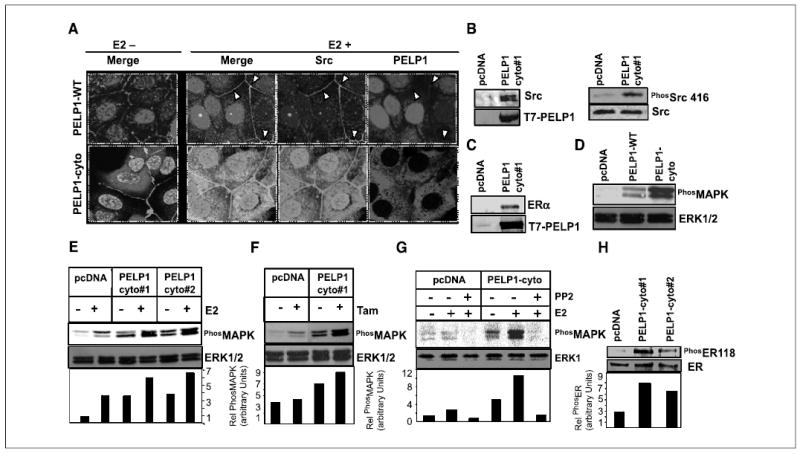
PELP1 cytoplasmic retention promotes PELP1-Src kinase interaction and MAPK activation. A, MCF-7 cells stably expressing T7-tagged PELP1-WT and PELP1-cyto were treated with or without E2 (10−9 mol/L) for 10 minutes. Cells were fixed, and the colocalization of PELP1 (red) and Src (green) was analyzed by confocal microscopy. Colocalization of PELP1 and Src is shown in yellow. B, pcDNA-and PELP1-cyto clones were cultured in 5% DCC serum. Total cellular lysates (2 mg) were subjected to immunoprecipitation using T7 antibody followed by Western blot analysis with Src antibody (left). Total lysate (100 μg) was analyzed by Western blotting using phospho-Src Tyr416 antibody (right). C, total cellular lysate (2 mg) from pcDNA or PELP1-cyto clones was subjected to immunoprecipitation by using T7 antibody followed by Western blot analysis with ERα antibody. D, MCF-7 clones expressing pcDNA, PELP1-WT, and PELP1-cyto were cultured in 5% serum, and total lysates were subjected to Western blotting by using phospho-MAPK antibody. Blots were stripped and reprobed with total MAPK as the loading control. E and F, MCF-7 clones expressing pcDNA or PELP1-cyto were treated with E2 (10−9 mol/L) or tamoxifen (10−8 mol/L) and total lysates were subjected to Western blotting by using a phospho-MAPK antibody. Blots were stripped and reprobed with total MAPK as the loading control. G, pcDNA and PELP1-cyto clones were pretreated with Src kinase inhibitor PP2 30 minutes before the addition of E2 (10−9 mol/L). Activation status of MAPK pathway was analyzed by Western blotting. H, MCF-7 clones expressing pcDNA and PELP1-cyto were cultured in 5% serum, and total lysates were subjected to Western blotting using phospho-ER Ser118 and subsequently reprobed with total ER.
