Figure 5.
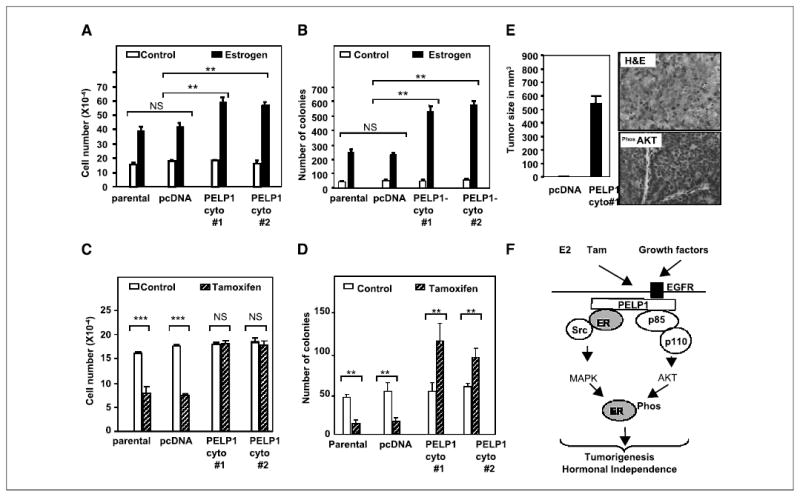
PELP1 deregulation promotes hormonal resistance and tumorigenesis. A and C, pcDNA- and PELP1-cyto–expressing clones were cultured in 5% DCC serum for 48 hours and treated with or without E2 (10−9 mol/L, A) or tamoxifen (10−8 mol/L, C) for 7 days, and the cell number was determined. B and D, the anchorage-independent growth potential of pcDNA- and PELP1-cyto–expressing clones was measured by the ability of the cells to form colonies on soft agar. Cells were treated with either E2 (10−9 mol/L, B) or tamoxifen (10−8 mol/L, D), and colonies were counted after 21 days. Similar results were obtained in three independent experiments. Columns, mean; bars, SE. **P < 0.01; ***P < 0.001; NS, not significant (Student’s t test). E, nude mice were injected in the mammary fat pad with 5 × 107 MCF-7 cells that stably express either pcDNA or PELP1-cyto, and tumor growth was measured after 8 weeks. Morphology of tumor was evaluated by H&E staining and activation of AKT pathway in the tumors was confirmed by immunohistochemistry with a phospho-AKT antibody. F, model for PELP1 regulation of ER functions. Cytoplasmic localization of PELP1 promotes its interactions with EGFR, Src kinase, and PI3K, thus leading to the activation of nongenomic pathways including MAPK and AKT. Such excessive activation of nongenomic pathways promotes hypersensitivity and tamoxifen resistance, which could in part be due to the modification of ER phosphorylation. PELP1 serves as an adaptor protein because it interacts with growth factor receptors, signaling kinases, and nuclear receptors; its deregulation may perturb ER signaling network toward tumorigenesis.
