Abstract
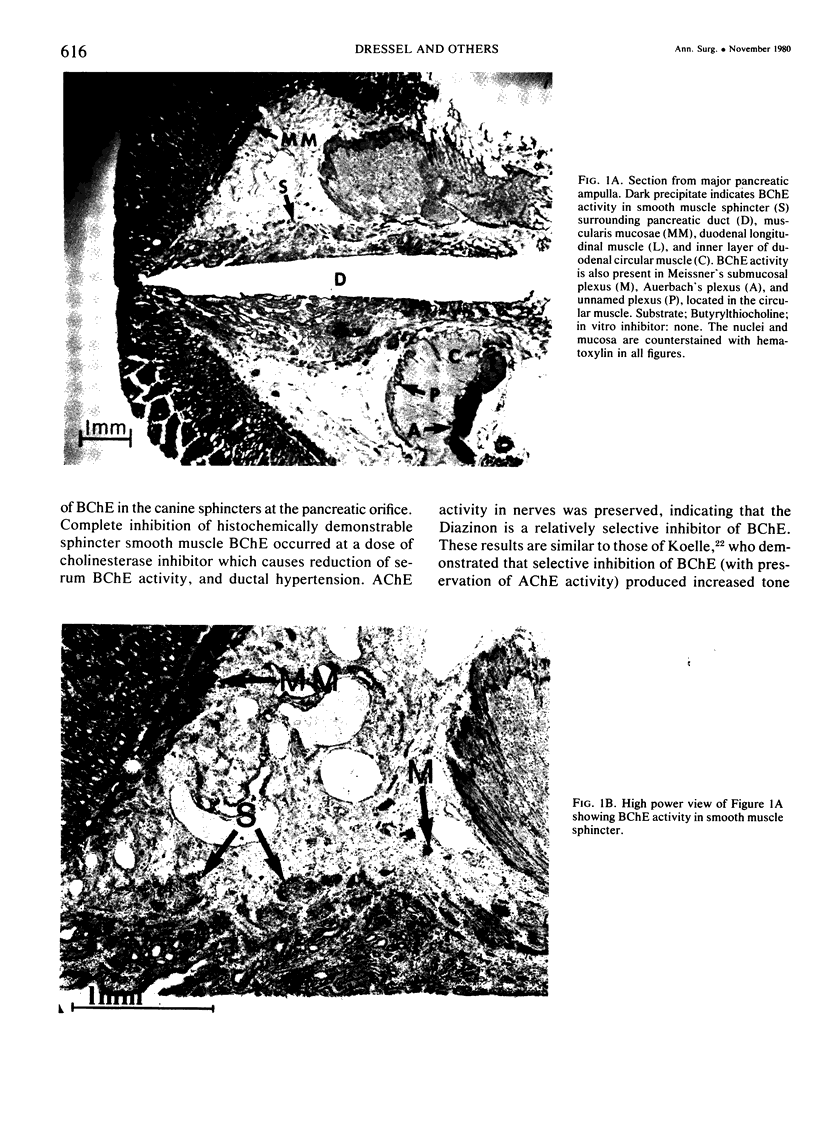
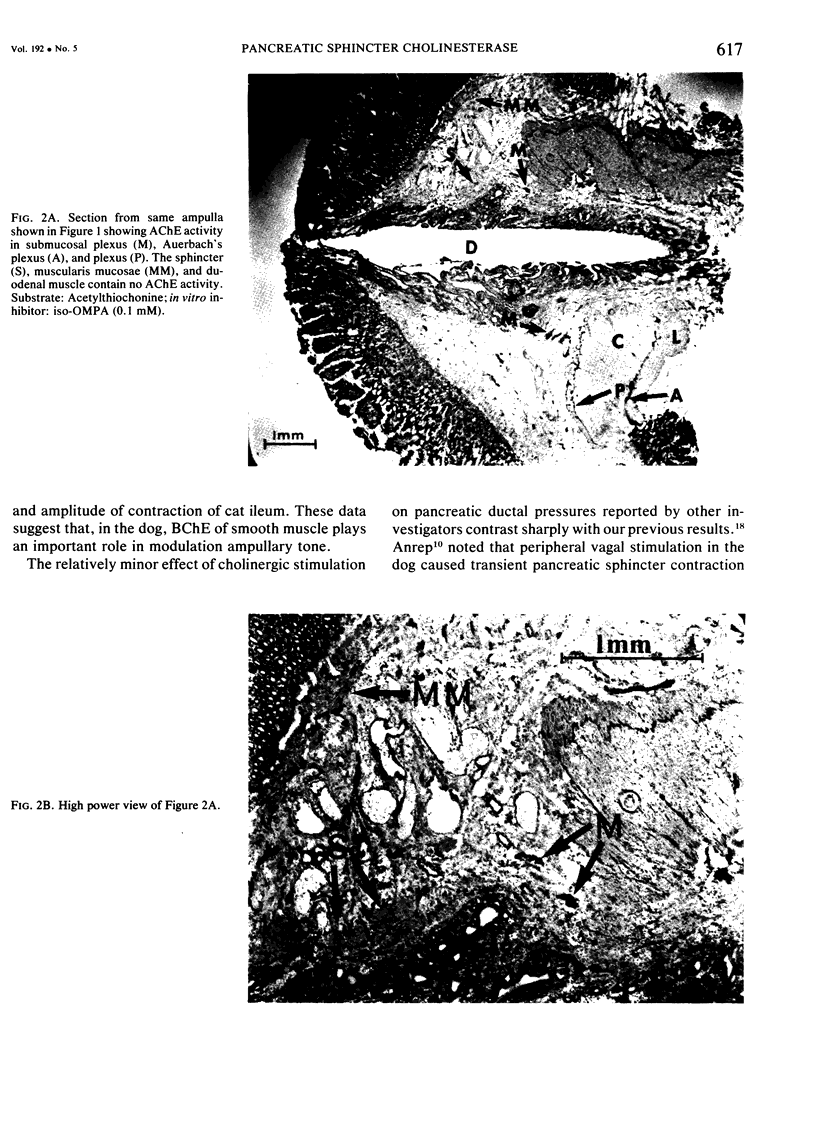
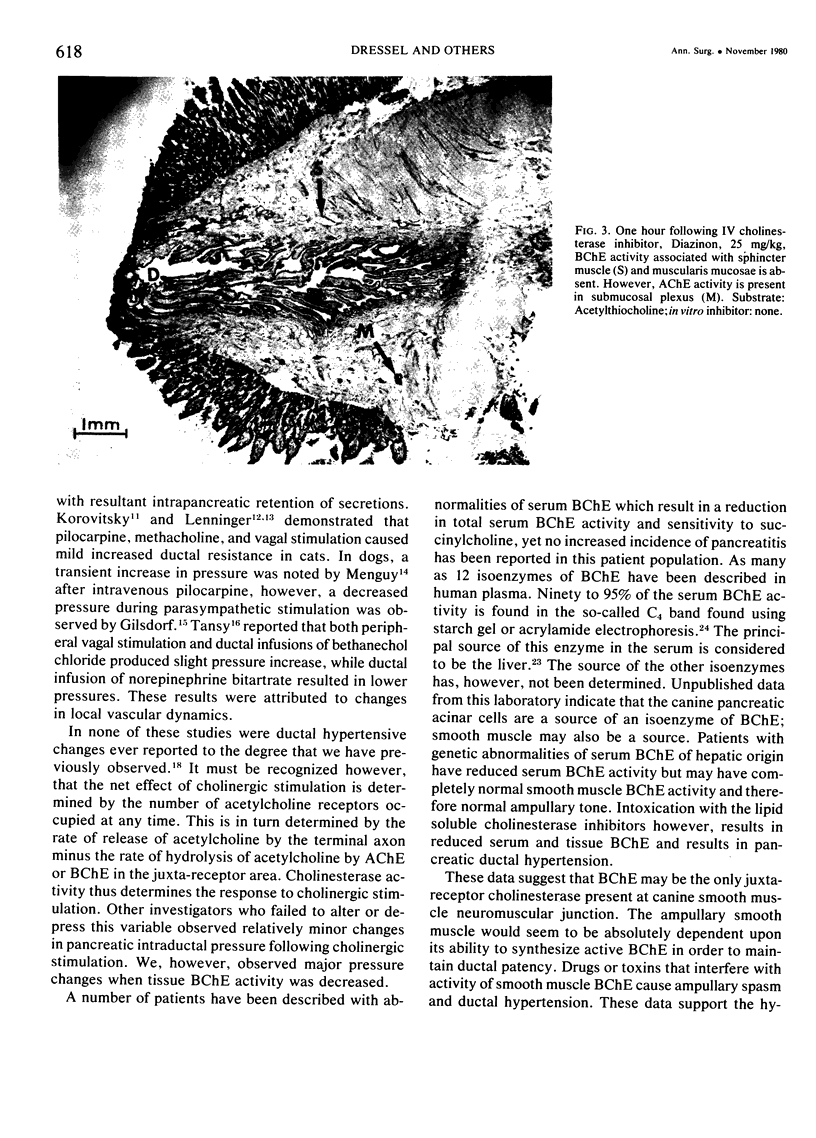
Previous work from this laboratory revealed in increased canine pancreatic intraductal pressure following cholinesterase inhibitor intoxication. The pressure was negatively correlated with serum butyrylcholinesterase (BChE) activity, suggesting that BChE activity mediated the pressure rise. This study uses a histochemical technique to investigate the tissue cholinesterase activity of the canine pancreatic sphincters and the effect of a cholinesterase inhibitor (ChEI) on tissue cholinesterase activity. In five control dogs, serial sections of the major and minor spincters were stained for acetylcholinesterase (AChE) and BChE activity. Four treated dogs were given the ChEI, O,O-diethyl-O- (2-isopropyl-6-methyl-4-pyrimidinyl) phosphoro-thioate, 25 mg/kg, one hour prior to excising the ampullae. In the control dogs, BChE activity is present in the periampullary nerves and the pancreatic smooth muscle sphincters. AChE activity is present in nerves but not in smooth muscle. In the treated group, following a dose of ChEI known to cause ductal hypertension, BChE activity was absent in the pancreatic sphincters but AChE activity was preserved in the periampullary nerves. These data suggest that the pancreatic ductal hypertension that occurs following ChEI administration is due to a selective reduction in pancreatic smooth muscle BChE activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anrep G. V. The influence of the vagus on pancreatic secretion: Second communication. J Physiol. 1916 Dec 15;50(7):421–433. doi: 10.1113/jphysiol.1916.sp001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN E. A. The choledocho- and pancreaticoduodenal junctions in the chimpanzee. Surgery. 1955 Jun;37(6):918–927. [PubMed] [Google Scholar]
- BOYDEN E. A. The choledochoduodenal junction in the cat. Surgery. 1957 May;41(5):773–786. [PubMed] [Google Scholar]
- Banks P. A. Acute pancreatitis. Gastroenterology. 1971 Sep;61(3):382–397. [PubMed] [Google Scholar]
- Bockman D. E., Schiller W. R., Anderson M. C. Route of retrograde flow in the exocrine pancreas during ductal hypertension. Arch Surg. 1971 Aug;103(2):321–329. doi: 10.1001/archsurg.1971.01350080237038. [DOI] [PubMed] [Google Scholar]
- Dressel T. D., Goodale R. L., Jr, Arneson M. A., Borner J. W. Pancreatitis as a complication of anticholinesterase insecticide intoxication. Ann Surg. 1979 Feb;189(2):199–204. doi: 10.1097/00000658-197902000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel T. D., Goodale R. L., Jr, Hunninghake D. B., Borner J. W. Sensitivity of the canine pancreatic intraductal pressure to subclinical reduction in cholinesterase acitivity. Ann Surg. 1979 Jul;190(1):6–12. doi: 10.1097/00000658-197907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICHHORN E. P., Jr, BOYDEN E. A. The choledochoduodenal junction in the dog; a restudy of Oddi's sphincter. Am J Anat. 1955 Nov;97(3):431–459. doi: 10.1002/aja.1000970305. [DOI] [PubMed] [Google Scholar]
- Gilsdorf R. B., Urdaneta L. F., Delaney J. P., Leonard A. S. Central nervous system influences on pancreatic secretion, sphincteric mechanisms, and blood flow and their role in the course of pancreatitis. Surgery. 1967 Oct;62(4):581–588. [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B., KOELLE E. S., FRIEDENWALD J. S. The effect of inhibition of specific and non-specific cholinesterase on the motility of the isolated ileum. J Pharmacol Exp Ther. 1950 Oct;100(2):180–191. [PubMed] [Google Scholar]
- Korovitsky L. K. The part played by the ducts in the pancreatic secretion. J Physiol. 1923 Mar 21;57(3-4):215–223. doi: 10.1113/jphysiol.1923.sp002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenninger S. Effects of parasympathomimetic agents and vagal stimulation on the flow in the pancreatic duct of the cat. Acta Physiol Scand. 1971 Jul;82(3):345–353. doi: 10.1111/j.1748-1716.1971.tb04975.x. [DOI] [PubMed] [Google Scholar]
- Lenninger S., Olin T. The effect of methacholine on the pancreatic duct of the cat studied with a radiological method. Scand J Gastroenterol. 1972;7(8):727–731. doi: 10.3109/00365527209180987. [DOI] [PubMed] [Google Scholar]
- MENGUY R. B., HALLENBECK G. A., BOLLMAN J. L., GRINDLAY J. H. Intraductal pressures and sphincteric resistance in canine pancreatic and biliary ducts after various stimuli. Surg Gynecol Obstet. 1958 Mar;106(3):306–320. [PubMed] [Google Scholar]
- Mendel B., Rudney H. Studies on cholinesterase: 1. Cholinesterase and pseudo-cholinesterase. Biochem J. 1943 Apr;37(1):59–63. doi: 10.1042/bj0370059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muensch H., Goedde H. W., Yoshida A. Human-serum cholinesterase subunits and number of active sites of the major component. Eur J Biochem. 1976 Nov 1;70(1):217–223. doi: 10.1111/j.1432-1033.1976.tb10972.x. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L. Acetylcholinesterase. Adv Enzymol Relat Areas Mol Biol. 1975;43:103–218. doi: 10.1002/9780470122884.ch3. [DOI] [PubMed] [Google Scholar]
- Tansy M. F., Salkin L. M., Innes D. L., Martin J. S., Landin W. E., Kendall F. M. The occlusive competence of the duodenal orifice of the canine pancreatic duct. Surg Gynecol Obstet. 1977 Jun;144(6):865–868. [PubMed] [Google Scholar]
- Tiscornia O. M., Martinez J. L., Sarles H. Some aspects of human and canine macroscopic pancreas innervation. Am J Gastroenterol. 1976 Oct;66(4):353–361. [PubMed] [Google Scholar]
- ZELANDER T., EKHOLM R., EDLUND Y. THE ULTRASTRUCTURE OF THE RAT EXOCRINE PANCREAS AFTER EXPERIMENTALLY OCCLUDED OUTFLOW. J Ultrastruct Res. 1964 Feb;10:89–102. doi: 10.1016/s0022-5320(64)90023-1. [DOI] [PubMed] [Google Scholar]







