Abstract
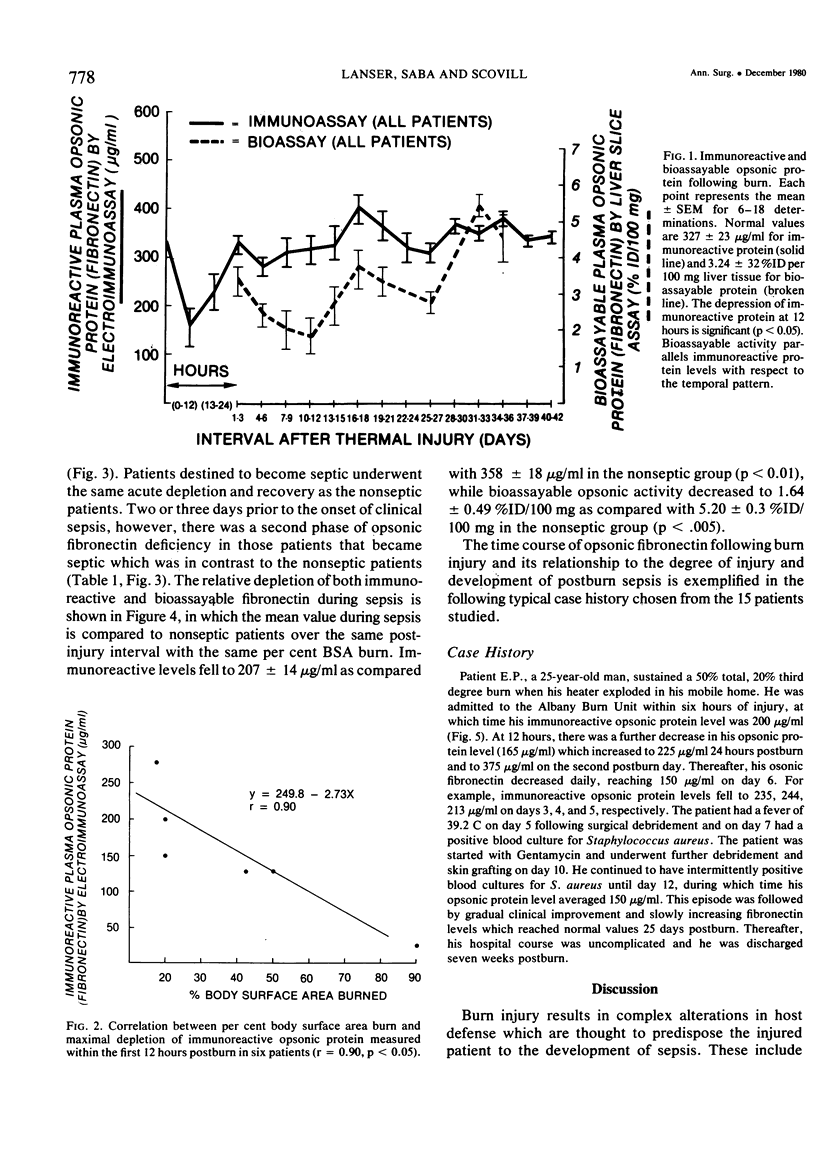
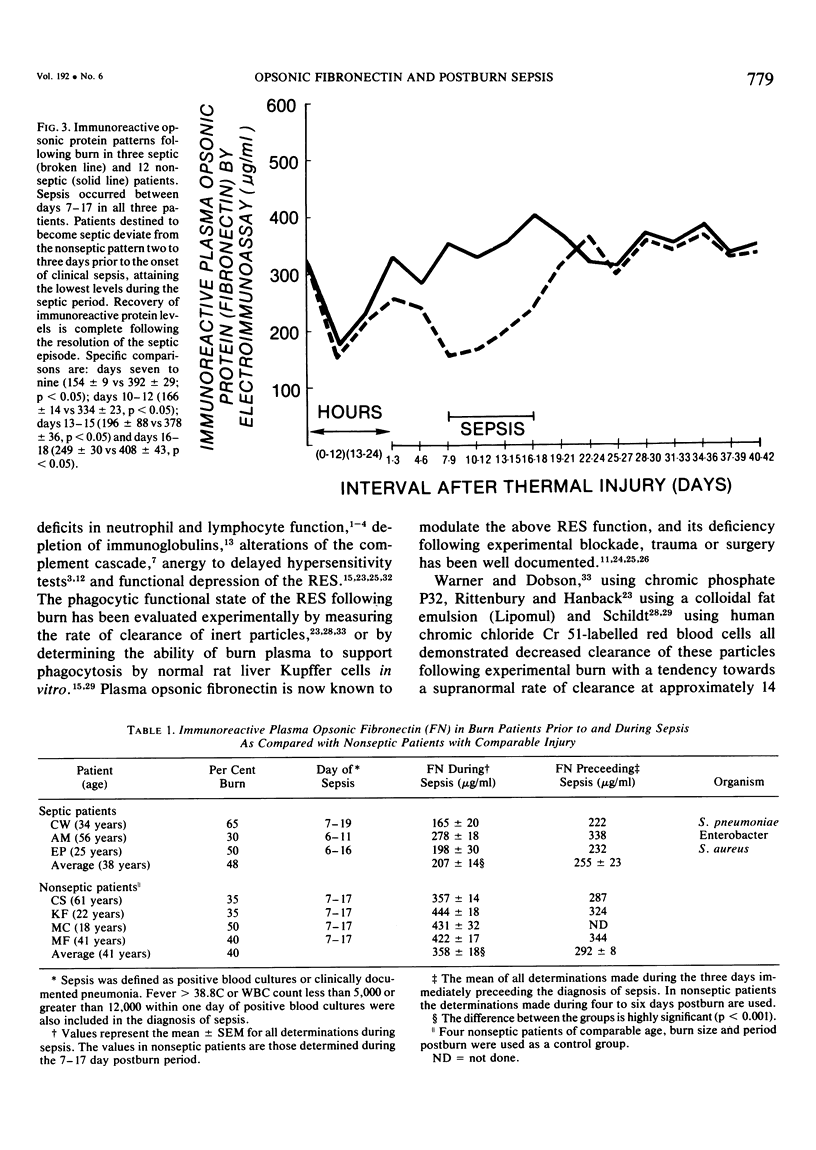
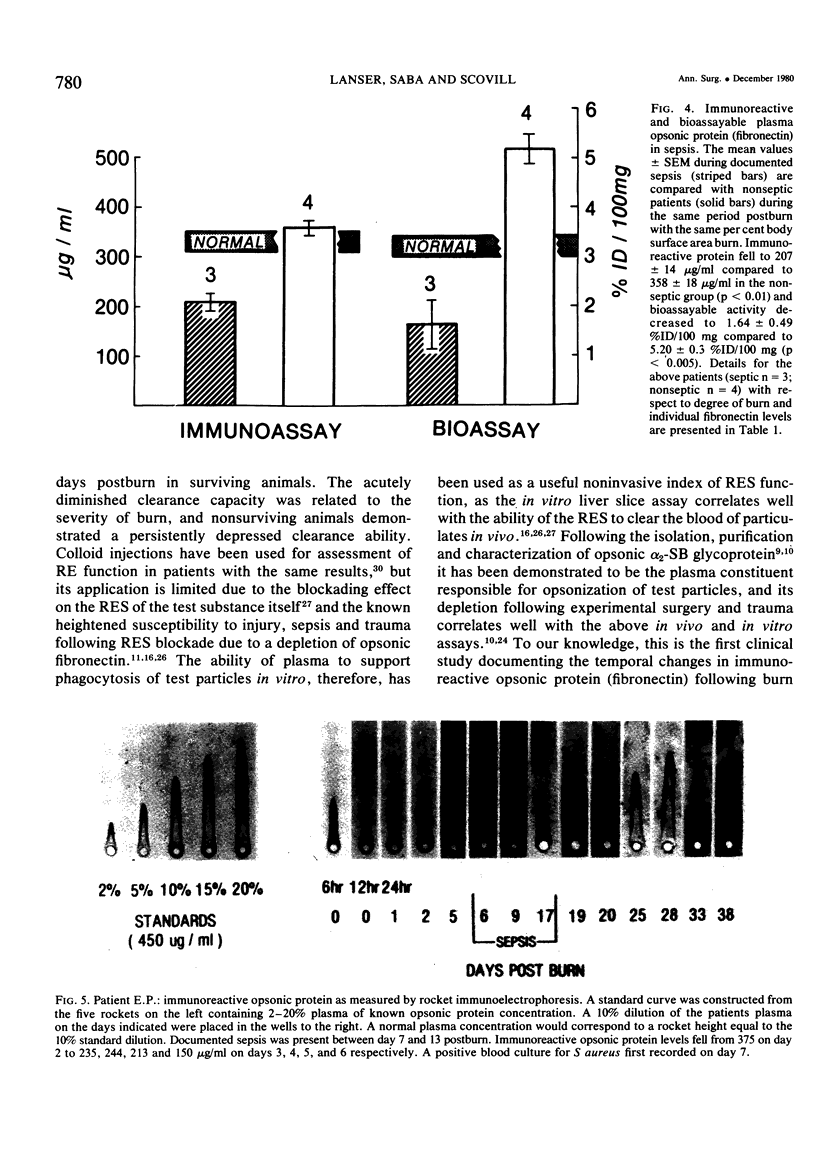
The time course of immunoreactive and bioassayable opsonic alpha 2-SB glycoprotein (plasma fibronectin), as well as its relationship to both the extent of injury and development of postburn sepsis, was evaluated following burn injury. Immunoreactive opsonic fibronectin was depleted acutely within hours following burn; its maximal depletion occurring 12 hours postburn injury. The magnitude of depletion was correlated with the body surface area burned, and normal levels were restored at 24 hours postinjury. There was a tendency toward rebound hyperopsonemia at two weeks postburn, with a slow return to normal over the ensuing weeks. Bioassayable opsonic protein levels, in general, paralleled those of immunoreactive protein. Following restoration of opsonic protein levels, a secondary phase of opsonic fibronectin deficiency (p equal to 0.05) developed in those burn patients that became septic. Moreover, this opsonic fibronectin deficiency actually became apparent prior to the onset of clinical sepsis, although it was maximal during sepsis. The resolution of the septic episode was associated with the return of plasma opsonic fibronectin levels to normal. The possibility that secondary deficiency in immunoreactive opsonic fibronectin may be a reliable index of impending sepsis following burn warrants further investigation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Meakins J. L. A physiological basis for the development of opportunistic infections in man. Ann Surg. 1972 Sep;176(3):273–287. doi: 10.1097/00000658-197209000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. W., Moncrief J. A. Alterations of the immune response following severe thermal injury. Arch Surg. 1966 Jul;93(1):75–83. doi: 10.1001/archsurg.1966.01330010077011. [DOI] [PubMed] [Google Scholar]
- Alexander J. W., Wixson D. Neutrophil dysfunction and sepsis in burn injury. Surg Gynecol Obstet. 1970 Mar;130(3):431–438. [PubMed] [Google Scholar]
- Altura B. M., Hershey S. G. Reticuloendothelial function in experimental injury and tolerance to shock. Adv Exp Med Biol. 1972;33(0):545–569. doi: 10.1007/978-1-4684-3228-2_57. [DOI] [PubMed] [Google Scholar]
- BALCH H. H. Resistance to infection in burned patients. Ann Surg. 1963 Jan;157:1–19. doi: 10.1097/00000658-196301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Altemeier W. A., Bjornson H. S. Complement, opsonins, and the immune response to bacterial infection in burned patients. Ann Surg. 1980 Mar;191(3):323–329. doi: 10.1097/00000658-198003000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell F. W. Pathophysiology of the respiratory distress syndrome. Arch Surg. 1974 Jan;108(1):44–49. doi: 10.1001/archsurg.1974.01350250036009. [DOI] [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P. An affinity method for the rapid purification of opsonic alpha 2 SB glycoprotein from serum. Adv Shock Res. 1979;2:55–71. [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P. Purification of alpha-2-opsonic protein from human serum and its measurement by immunoassay. J Reticuloendothel Soc. 1978 Feb;23(2):119–134. [PubMed] [Google Scholar]
- Casson P., Solowey A. C., Converse J. M., Rapaport F. T. Delayed hypersensitivity status of burned patients. Surg Forum. 1966;17:268–270. [PubMed] [Google Scholar]
- Daniels J. C., Larson D. L., Abston S., Ritzmann S. E. Serum protein profiles in thermal burns. I. Serum electrophoretic patterns, immunoglobulins, and transport proteins. J Trauma. 1974 Feb;14(2):137–152. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Goldman A. S., Rudloff H. B., McNamee R., Loose L. D., DiLuzio N. R. Deficiency of plasma humoral recognition factor activity following burn injury. J Reticuloendothel Soc. 1974 Mar;15(3):193–198. [PubMed] [Google Scholar]
- Kaplan J. E., Saba T. M. Humoral deficiency and reticuloendothelial depression after traumatic shock. Am J Physiol. 1976 Jan;230(1):7–14. doi: 10.1152/ajplegacy.1976.230.1.7. [DOI] [PubMed] [Google Scholar]
- LEE L., McCLUSKEY R. T. Immunohistochemical demonstration of the reticuloendothelial clearance of circulating fibrin aggregates. J Exp Med. 1962 Nov 1;116:611–618. doi: 10.1084/jem.116.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEDBERG C. F. Antibacterial resistance in burns. II. The effect on unspecific humoral defense mechanisms, phagocytosis. and the development of bacteremia. An experimental study in the guinea pig. Acta Chir Scand. 1961 Jun-Jul;121:351–358. [PubMed] [Google Scholar]
- MCRIPLEY R. J., GARRISON D. W. INCREASED SUSCEPTIBILITY OF BURNED RATS TO PSEUDOMONAS AERUGINOSA. Proc Soc Exp Biol Med. 1964 Feb;115:336–338. doi: 10.3181/00379727-115-28906. [DOI] [PubMed] [Google Scholar]
- Munster A. M. Alterations of the host defense mechanism in burns. Surg Clin North Am. 1970 Dec;50(6):1217–1225. doi: 10.1016/s0039-6109(16)39282-9. [DOI] [PubMed] [Google Scholar]
- Rittenbury M. S., Hanback L. D. Phagocytic depression in thermal injuries. J Trauma. 1967 Jul;7(4):523–540. doi: 10.1097/00005373-196707000-00004. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Scovill W. A., Bernard H. Cryoprecipitate reversal of opsonic alpha2-surface binding glycoprotein deficiency in septic surgical and trauma patients. Science. 1978 Aug 18;201(4356):622–624. doi: 10.1126/science.675246. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980 Apr;68(4):577–594. doi: 10.1016/0002-9343(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Schildt B. E., Bouveng R. Effect of burning on hepatic phagocytosis as studied in vitro. Life Sci I. 1971 Apr 1;10(7):397–404. doi: 10.1016/0024-3205(71)90145-7. [DOI] [PubMed] [Google Scholar]
- Schildt B. E. Function of the RES after thermal and mechanical trauma in mice. Acta Chir Scand. 1970;136(5):359–364. [PubMed] [Google Scholar]
- Schildt B., Gertz I., Wide L. Differentiated reticuloendothelial system (res) function in some critical surgical conditions. Acta Chir Scand. 1974;140(8):611–617. [PubMed] [Google Scholar]
- Scovill W. A., Annest S. J., Saba T. M., Blumenstock F. A., Newell J. C., Stratton H. H., Powers S. R. Cardiovascular hemodynamics after opsonic alpha-2-surface binding glycoprotein therapy in injured patients. Surgery. 1979 Aug;86(2):284–293. [PubMed] [Google Scholar]
- Scovill W. A., Saba T. M., Blumenstock F. A., Bernard H., Powers S. R., Jr Opsonic alpha2 surface binding glycoprotein therapy during sepsis. Ann Surg. 1978 Oct;188(4):521–529. doi: 10.1097/00000658-197810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER G. F., DOBSON E. L. Disturbances in the reticulo-endothelial system following thermal injury. Am J Physiol. 1954 Oct;179(1):93–98. doi: 10.1152/ajplegacy.1954.179.1.93. [DOI] [PubMed] [Google Scholar]