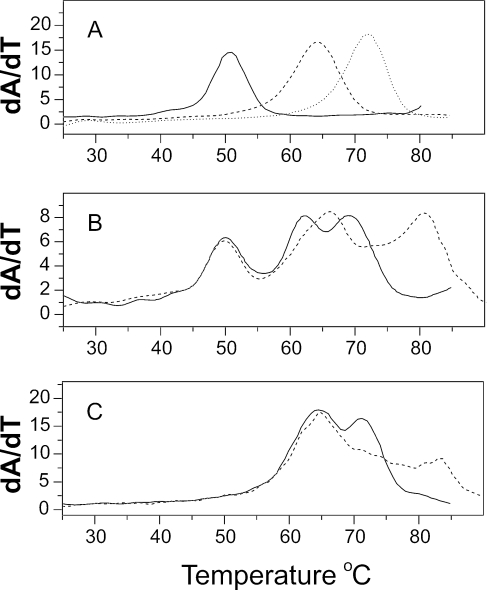
Figure 3.
Melting of deoxyoligonucleotide mixtures. (A). Melting of (T2G20T2)• (T2C20T2) (black line), (T2(AC)10T2)• (T2(GT)10T2) (dashed line) and (T2(AG)10T2)• (T2(CT)10T2) (dotted line). All samples were at 2 µM duplex concentration. (B). Melting of a mixture of the three deoxyoligonucleotides in (A) in the absence (black line) or presence (dashed line) of the bisanthracycline WP631 (32). Each deoxynucleotide in the mixture was at a concentration of 0.67 µM duplex, for a total duplex concentration of 2 µM. WP631 was add to a final concentration of 1.6 µM. (C). Melting of a binary mixture (4 µM total duplex) of (T2(AC)10T2)• (T2(GT)10T2) and (T2(AG)10T2)• (T2(CT)10T2) alone (black line) or in the presence of 1.6 µM WP631 (dashed line).