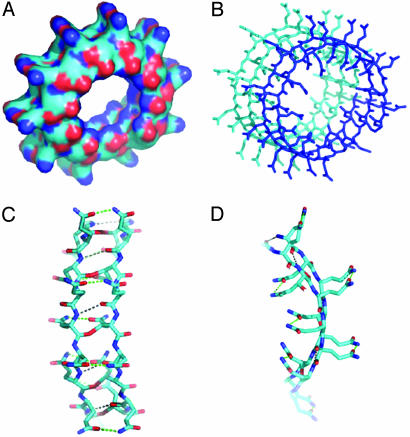
Fig. 1.
Computer renderings of the Perutz et al. (5) structure of poly Q. (A) Space-filling model of one subunit of 42 Q residues with two turns of β-strands, showing the central pore, here tilted, that is perpendicular to the plane of the membrane. Note the outer convolutions of the surface of the subunit, by the outward-facing Q residues. These might serve to make gear-like associations between β-amyloid subunits in the plane of the membrane; others might serve as regions of attachment of specific integral membrane proteins to the membrane-interior regions of the poly Q amyloid, such as we propose in the text. Red balls are O atoms, and blue ones are N atoms. (B) Stick model of two stacked subunits of poly Q, each subunit containing 42 Q residues, one subunit rendered in dark blue, the other in light blue. (C) Stick model of a short segment of one subunit (enlarged) that faces a region of bonding between one turn and another of the subunit, showing the hydrogen bonds (strings of dots) formed between backbone 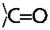 (red) and
(red) and 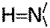 (dark blue) groups. (D) Stick model of a small segment of one subunit, showing (by strings of dots) the hydrogen bonds formed by (i) inter-β-strand bonds between the backbone
(dark blue) groups. (D) Stick model of a small segment of one subunit, showing (by strings of dots) the hydrogen bonds formed by (i) inter-β-strand bonds between the backbone 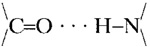 groups, (the O in red and the N in dark blue) and (ii)
groups, (the O in red and the N in dark blue) and (ii) 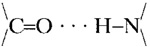 of the alternate Q residues extending from one or the other surface of the two-turn helix sheet. This view is parallel to the axis that runs down the pore of the subunit, and perpendicular to the plane of the membrane bilayer.
of the alternate Q residues extending from one or the other surface of the two-turn helix sheet. This view is parallel to the axis that runs down the pore of the subunit, and perpendicular to the plane of the membrane bilayer.