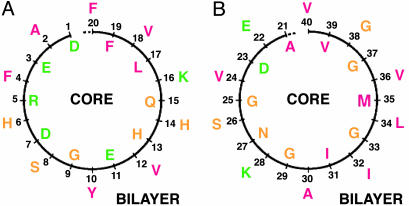
Fig. 3.
A schematic view of the distribution of the amino acid residues of Aβ 1-40 in projection along the first (A) and second (B) turns of the Perutz et al. subunit, perpendicular to the axis running down the water-filled core. Successive residues (numbered) face into the core or into the membrane bilayer. The residues are colored red if they are predominantly hydrophobic, i.e., A, F, I, L, M, V, and Y); green if, at pH 7, they are predominantly hydrophilic; and yellow if they are in between. For the first turn (A), the numbers of each type of residue facing into the bilayer are six hydrophobic, one hydrophilic (i.e., D, E, K, and R), and three in-between. The residues facing into the water-filled core are two hydrophobic, five hydrophilic (four in a single sequence DERD), and three in between. For the second turn (B), the residues facing into the bilayer are six hydrophobic, two hydrophilic, and two in between. The residues facing into the water-filled core are four hydrophobic, one hydrophilic, and five in between. Generally, therefore, there are more hydrophobic than hydrophilic (plus in between) residues facing the bilayer, and more hydrophilic (plus in between) than hydrophobic residues facing the water-filled core in each turn, as would be expected thermodynamically. The single-letter symbols are used for the amino acid residues. A, alanine; D, aspartic acid; E, glutamic acid; F, phenylalanine; G, glycine; H, histidine; I, isoleucine; K, lysine; L, leucine; M, methionine; N, asparagine; Q, glutamine; R, arginine; S, serine; V, valine; Y, tyrosine.